CHAPTER 91 Vascular Malformations of the Spinal Cord
Vascular lesions of the spinal cord are a rare cause of neurologic dysfunction, representing less than 5% of all intraspinal pathology.1 This heterogeneous class of entities encompasses a wide range of etiologic, anatomic, pathophysiologic, and clinical features. They occur throughout the spine and may affect any age group, although the vast majority present between the third and fifth decades of life.1 Symptoms and signs result from ischemia, venous congestion, hemorrhage, or mechanical compression of the spinal cord and roots. Most spinal vascular lesions are characterized by an abnormal arteriovenous shunt, which may be located within the dura, on the spinal cord surface, within the substance of the spinal cord, or rarely extradurally.2–5 The shunt may take the form of a simple direct arteriovenous fistula (AVF) or of a more complex nidus of dysmorphic arteries and veins without an intervening capillary bed. Whereas the latter lesions are typically congenital, the more common fistulous lesions are often acquired.6
Classification
Various nomenclature and classification systems have been employed in the description of spinal vascular lesions.4,7–9 As a first approximation, these lesions can be broadly divided by the presence or absence of an arteriovenous shunt. Those lesions without a shunt include cavernous malformation (a lesion of capillary structure10) and, possibly, hemangioblastoma.4 Spinal cord arteriovenous shunts, on the other hand, have traditionally been separated into four types on the basis of the location and angioarchitecture of the abnormal arteriovenous connection (Box 91–1). Types I and IV represent direct AVFs that occur within the dural root sleeve (type I) or on the spinal cord surface (type IV). Type II arteriovenous malformations (AVMs) are true congenital malformations, similar to their intracranial pial counterparts. Type III AVMs are also congenital but demonstrate extensive contiguous involvement of intramedullary, intradural-extramedullary, and extradural/paraspinal tissues.
Type I
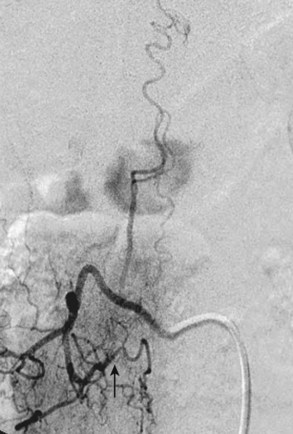
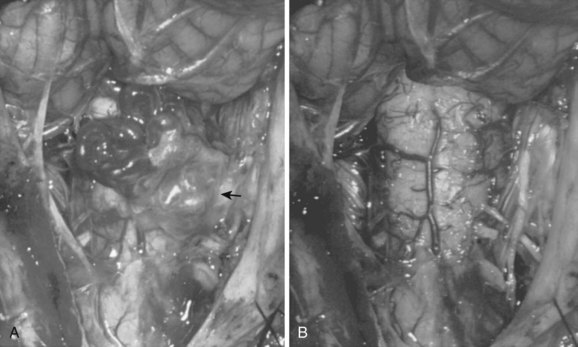
Type I AVFs, the most commonly occurring type of spinal vascular malformation, have also been termed long dorsal AVMs; single coiled vessel AVMs; angioma venosum racemosum; dural AVF; micro-AVF; and, more recently, intradural dorsal AVF.4,7,8,11–17 A more sophisticated understanding of the anatomy and pathophysiology of these fistulas has developed since the seminal description by Wyburn-Mason,17 who characterized these as purely venous lesions. The advent of selective spinal angiography in the 1960s reclassified them as slow-flow AVFs. Early surgical treatment was directed at stripping the long dorsal vein off the spinal cord surface. It was assumed that tiny feeding vessels, too small to be seen angiographically, supplied this dilated vein throughout its length. This treatment approach did stabilize or improve symptoms in some patients, but postoperative neurologic deterioration was seen in many others. Kendall and Logue,13 in 1977, correctly recognized these lesions as simple AVFs between a radicular or radiculomedullary artery and a medullary vein, with the fistulous connection located in the dural root sleeve (Fig. 91–1). The entire intradural portion of the malformation, therefore, represents the enlarged spinal cord venous system that has been pathologically engorged from retrograde flow from the fistula into the spinal cord veins (Fig. 91–2). These malformations are likely often acquired and arise predominantly at thoracic and thoracolumbar levels. As mentioned earlier, these shunts and their venous drainage are almost universally dorsally located with ventral communications being exceedingly rare.18
Type II
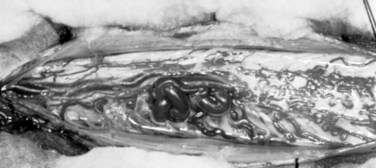
These malformations are known as glomus, nidus, or simply intramedullary type AVMs.4,7 They are angiographically and operatively well-defined lesions consisting of a distinct conglomeration of dysmorphic arteries and veins in direct communication without an intervening capillary bed (Figs. 91-3 and 91-4).19,20 The location of the nidus may be completely or partially intramedullary and only rarely is confined to the epipial tissue of the cord. These latter lesions are sometimes referred to as perimedullary type II AVMs and most commonly occur dorsally at the cervicomedullary junction (Fig. 91–5).
Typical type II AVMs may arise anywhere within the spinal cord but predominantly are found at the cervical and lumbar enlargements or the conus medullaris. Type II AVMs of the cervical spinal cord frequently have multiple feeding vessels of anterior spinal, posterior spinal, and radiculomedullary arteries, whereas type II AVMs of the thoracic spinal cord or conus are often supplied via a single enlarged branch of the anterior spinal artery. Latent anastomotic channels invariably exist, however, which may emerge after proximal ligation or endovascular occlusion of the primary feeding vessel (Fig. 91–6).
Type III
Also known as juvenile or metameric AVMs, type III malformations are fortunately rare. These lesions do not possess a discrete nidus but rather consist of diffuse arteriovenous shunts with variable degrees of involvement of the spinal cord, vertebral, and paraspinal tissues (Fig. 91–7). Other metameric anomalies of associated organs and the skin are commonly associated with these lesions.
Type IV
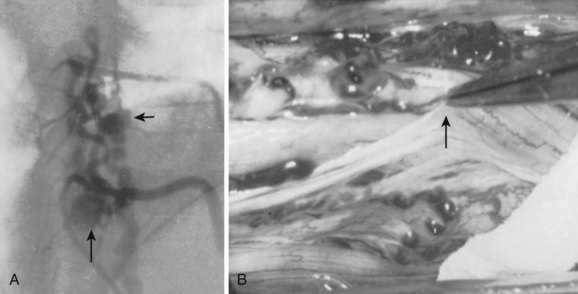
Type IV malformations are completely intradural fistulas, variably referred to as perimedullary AVF, macro-AVF, or intradural ventral AVF.4,7,8,12,21,22 Most occur in the thoracolumbar region as a fistula between the anterior spinal artery (ASA) and vein (ASV) on the ventral spinal cord surface. Gueguen and colleagues23 as well as Anson and Spetzler12 subdivided these arteriovenous shunts according to the complexity and size of the lesion. A type IV-A malformation is a small, simple direct fistula with a single ASA feeder. Type IV-B lesions are medium-sized fistulas and have additional, smaller feeding vessels arising from either the ASA or posterior spinal artery (PSA). Type IV-C fistulas are giant sized and demonstrate several enlarged ASA and PSA feeding branches with dilated venous outflow. The subtypes may represent progressive changes resulting from venous congestion, thrombosis, collateral vessel recruitment, or ischemia. The sporadic nature of these malformations suggests an acquired rather than congenital nature, but their rarity makes their etiology difficult to determine.
Pathophysiology and Symptomatology
Type I
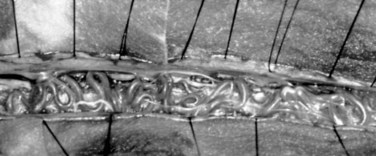
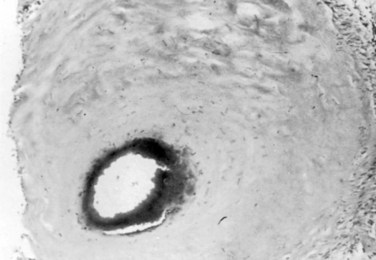
Type I AVFs produce progressive spinal cord ischemia from venous hypertension.7,11,15,16,24–28 Despite slow flow through the shunt, intradural venous pressures become markedly elevated and may approach systemic mean arterial pressure. Because spinal cord perfusion pressure is equal to mean systemic arterial pressure minus venous pressure, progressive spinal cord ischemia may ensue. The elevation of intraspinal venous pressures during activity or exercise accounts for the reversible ischemic symptoms often seen in these patients. Venous hypertension may be further exacerbated by structural changes such as reductions in venous diameter and compliance secondary to intimal thickening and hyalinization seen with chronic exposure to high intravenous pressures (Fig. 91–8). Episodic acute neurologic deterioration in these patients may occur as a result of venous thrombosis. In rare cases, patients may present with subarachnoid hemorrhage mimicking that of intracranial aneurysm rupture.29,30
It follows then that type I AVFs produce symptoms usually in middle and advanced adult years with a mean age at symptom onset around 50 years. Men are four to five times more commonly affected than women. The majority occur in the thoracic or thoracolumbar region. Symptoms typically arise insidiously with back and leg pain and mild sensorimotor dysfunction. In early stages, symptoms may mimic those of neurogenic claudication secondary to spinal stenosis. Neurologic examination, however, often reveals mixed upper and lower motor neuron disease and patchy sensory loss, clearly differentiating the clinical picture from degenerative lumbar stenosis. The natural history of type I AVFs produces an inexorable progression of symptoms occasionally punctuated by episodes of acute worsening, as in the classic Foix-Alajouanine syndrome. If untreated, these lesions lead to significant disability and wheelchair dependence in most patients within 6 months to 3 years after symptom onset.15
Numerous investigators have demonstrated that preoperative neurologic status is the most important predictor of post-treatment outcomes.28,31–33 Median time from symptom onset to diagnosis in modern series ranges from 15 to 23 months.32–36 If type I AVFs are treated early in their course, symptoms are often reversible. However, in more chronic cases, progressive ischemia ultimately leads to irreversible neuronal loss and infarction. Therefore definitive treatment should be accomplished as early in the disease as possible because function is unlikely to improve in the presence of severe incapacity.
Type II
Type II AVMs present in childhood or adult years. An acute presentation from subarachnoid hemorrhage or intramedullary hemorrhage is most common.16 As mentioned earlier, the presence of venous aneurysms seems to increase the risk of a hemorrhagic event. The acute onset of severe neck or back pain (“coup de poignard”37) approximates the level of the malformation and is typically the first symptom of AVM hemorrhage. The occurrence and progression of neurologic deficit are variable and dependent on the location and severity of the hemorrhage. For example, typical headache and nuchal rigidity may be the only symptoms of a spinal subarachnoid hemorrhage and usually lead to the standard evaluation for an intracranial ruptured aneurysm.38 On the other hand, objective deficits are typically seen with intramedullary hemorrhage. These deficits evolve over minutes to hours, and some degree of recovery is usually seen after incomplete lesions.
Types III and IV
Similar to type II AVMs, these high-flow lesions produce symptoms due to vascular steal/ischemia, hemorrhage, and mass effect. Type III AVMs have been associated with genetic syndromes such as Klippel-Trenaunay-Weber.39 Type IV lesions have an equal incidence in males and females. Symptoms may arise at any age, although most patients present in childhood and early to middle adult years. Associations with hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber disease) and Kartagener syndrome have been noted in younger patients.7,8,40,41
Radiology
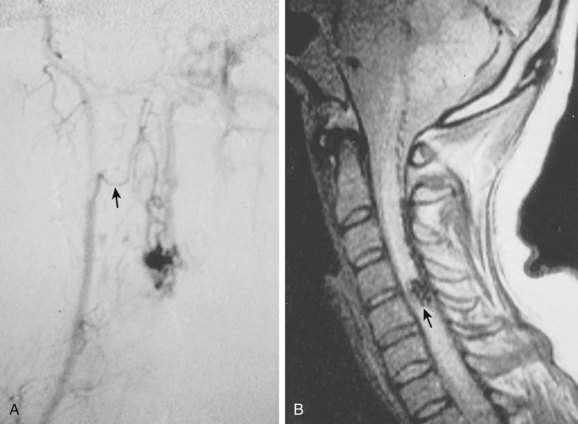
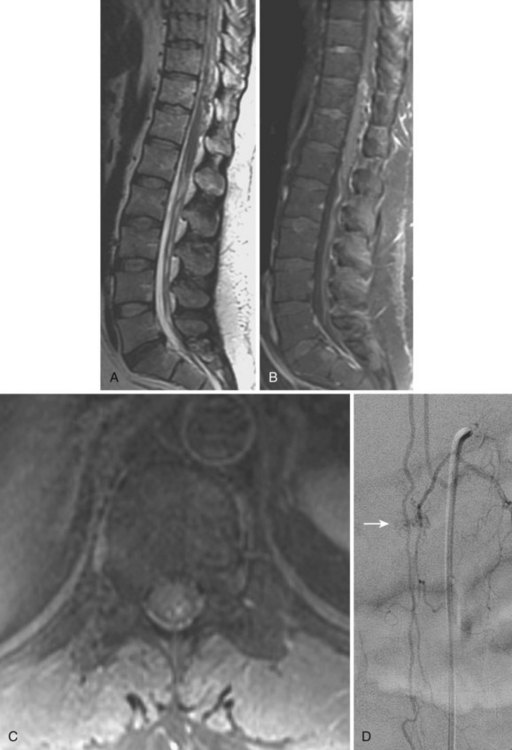
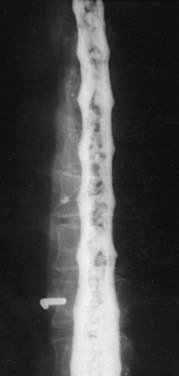
Selective spinal angiography remains the gold standard for the definitive diagnosis and characterization of spinal vascular malformations. This imaging study identifies the locations and flow characteristics of vascular shunts, as well as the sites of critical radiculomedullary arteries (e.g., artery of Adamkiewicz).13,20–22,42–46 Its inherently invasive nature, however, has led to the development and use of alternative modalities as screening procedures. These currently include magnetic resonance imaging (MRI), magnetic resonance angiography (MRA), computed tomographic (CT) angiography (CTA), and CT myelography. Myelography has a long history in the diagnosis of spinal AVMs, particularly for type I AVFs in which the characteristic serpentine filling defect on the dorsal spinal cord surface is often apparent (Fig. 91–9). These enlarged vessels are commonly identified on screening magnetic resonance scans. (Fig. 91–10). If the filling defect extends toward a neural foramen, the level and side of the fistula may further be suggested. The dilated venous filling defect should be differentiated from the venous dilatation seen rostral to high-grade extradural stenotic blocks.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree