CHAPTER 96 Vascular Complications in Spinal Surgery
“The surgeon who says he has no complications is either a nonoperative one or a liar.”
— The Johns Hopkins Hospital Reports 19:71, 1920
“Prevention is better than cure.”
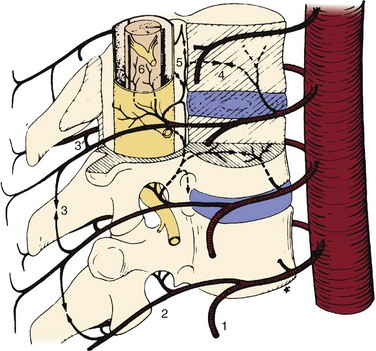
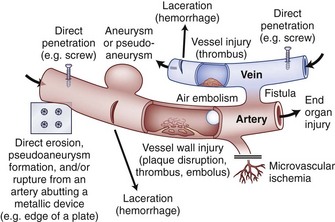
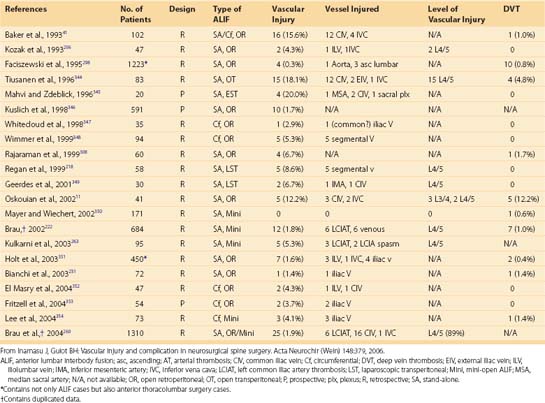
Vascular complications associated with spinal surgery are increasing in frequency, due primarily to the rise in open and endoscopic anterior procedures for the thoracolumbar spine, as well as a general increase in documentation of spine surgeries in spine periodicals.1–21 This increase is from 1997-2003, reported to be more than 400% by Ikard.22 A vascular complication may be defined as an injury to a blood vessel and its sequelae, which result directly or indirectly from a surgical approach, procedure, or operative technique. Certain conditions like neuromuscular scoliosis, Ehlers Danlos, and Marfan syndrome have increased bleeding associated with their surgeries. This is also true of patients taking aspirin, nonsteroidal anti-inflammatories, clopidogrel, and many herbals and over-the-counter medications.23 For the purposes of this chapter, a vascular complication may arise directly from vascular injury or indirectly as a consequence of the injury. Mechanisms of blood vessel injury are (1) laceration, (2) traction, (3) malpositioned instrumentation, and/or (4) compression.24 The spectrum of manifestations of vascular injuries is summarized as (1) hemorrhage from a laceration of a vessel wall25; (2) vessel wall injury resulting in an aneurysm or arteriovenous fistula26; (3) blood flow consequences at the macroscopic end organ level, or ischemia on a microscopic level13; and (4) internal vessel problems such as embolization (air, plaque, or clot); and (5) thrombosis or direct injury from instrumentation like malpositioned screws or erosions from instrumentation in contact with pulsatile major arteries (Fig. 96–1).27
Vascular complications in spinal surgery carry with them potentially dire circumstances including death. As with any complication, the best treatment is prevention. Most vessel injuries are reversible; however, prompt recognition and management are essential. Prevention of vascular complications is assisted by (1) knowledge of the vascular anatomy and common variants,28,29 (2) knowledge of the blood vessel and bone relationships,30,31 (3) gentle intraoperative techniques with appropriate illumination and magnification,17 and (4) preoperative planning.17 This chapter stresses the relations of vascular anatomy to the spine and emphasizes the spectrum of spinal vascular injuries, their prevention, and early recognition. The most common vascular complication in spinal surgery is direct laceration of the vessel resulting in acute bleeding. In reality, vessels are lacerated in every spinal surgery and are an ever-present problem affecting two of the three key preventative principles of spinal surgery (hemostasis, adequate exposure with illumination and visualization). For example, in a surgery of 5641 cases, Neo reported an incidence of vertebral artery injuries to 0.14%.32 Vascular complications are underreported and true incidences are impossible to know. A further illustrative example of this “underreporting” would be a laceration of the left common iliac vein occurring during an anterior lumbar spinal exposure at L4-L5. The vascular and spinal surgeons recognize the injury and rapidly isolate and repair it. Although intraoperative blood loss is increased, there are no other sequelae. Some prominent surgeons do not consider this a “complication” but rather a routine exposure risk of the lumbosacral spine. Thus this is neither noted nor reported. Those vascular complications that can be encountered are delineated by vessel anatomic level and injury type with mechanism of injury in parenthesis in Table 96–1.
TABLE 96–1 Reported Vascular Complications in Spinal Surgery
| Cervical Spine | Thoracic Spine | Lumbar Spine |
|---|---|---|
Abdominal aorta (direct injury) |
Three primary implications of vascular complications in spinal surgery exist: (1) the consequences and sequelae of brisk, prohibitive bleeding (i.e., hemorrhagic shock)33; (2) interruption of vascular supply to vital organs such as the spinal cord or brainstem34–36; or (3) secondary injury as a result of inadequate visualization caused by bleeding.17,37 These complications vary depending on the area of the spine involved and the surgical approach used.38 These are not mutually exclusive and often occur in series or combination. The former category is self-explanatory, resulting from injury to, or disruption of, major blood vessels adjacent to the vertebral bodies. In the second category, complete neurologic dysfunction or organ failure (reperfusion syndrome—see “Anterior Lumbar Vascular Complications” later) can occur. The last category is illustrated by a spinal cord or nerve root injury resulting from inadequate hemostasis and poor visualization from inadequate control of bleeding. Each of these categories has real and theoretical sequelae that may be irreversible and permanent where time is the most important singular factor. Furthermore, this chapter discusses vascular complications by anatomic area (anterior and posterior exposures), as well as other vascular-related complications such as superior gluteal artery injury during posterior iliac crest bone graft harvest and deep vein thrombosis (DVT).
Inamasu and Guiot, neurosurgeons, have further defined and classified areas of vascular risks during spinal surgery.39 Although similar to our approach, these also consider certain procedures and their anatomic location. Clearly, vascular complications are increasing in the cervical and lumbar spine due to (1) better reporting; (2) increased numbers of cases in the upper cervical spine where lateral mass C1 screws place at risk the vertebral artery and internal carotid artery and C1-C2 transarticular screws, which risk the vertebral artery; (3) marked increased posterior cervical lateral mass fixation, which now has important details to avoid injury to the vertebral artery using techniques other than that of Magerl (1.3% incident reported by Neo)32; and (4) increased procedures and techniques requiring anterior, anterolateral, or direct anterior retroperitoneal lumbar exposures that require an ever-increasing role of all anterior surgery (e.g., anterior lumbar interbody fusion, total disc arthroplasty, a plethora of procedures for discogenic pain and complex spinal deformities).
Also, the number of vascular complications has increased with the paradigm shift to total disc replacement both in the early postimplantation period, as well as in longer follow-up (Table 96–2). These approaches are using a straight rectus-splitting anterior retroperitoneal approach exposing L3 to S1. At L5-S1 the left common iliac vein is primarily at risk, and at L4-5 and L3-4 the vena cava and aorta along with their branches of the common iliac artery and vein are at risk. The recurrent or ascending iliolumbar vein is a real source of hemorrhage coming off the common iliac vein posterior or posterolaterally for L4-5 exposures. Although venous lacerations are still most common, more arterial complications, especially occlusions and thromboses, are being reported.
Potentially devastating vascular complications in these cases involving the left common iliac artery can result in rhabdomyolysis and loss of limb or life (multiple organ dysfunction, MODs, a part of a reperfusion syndrome), and a left common iliac vein can thrombose, especially if suture repair is required, significantly altering its diameter and/or flow. The single most important identifiable reason for increased vascular complications is the increased number of anterior spinal cases from the neck to the sacrum and pelvis. This has definitely been noted in multiple studies. It probably started around 1990 and did not became visible in the literature because of publication and writing delays until about 1995.22 Current efforts include safety based on evidence-based protocols.40 This is clearly the wave of the future.
Cervical Spine
Anterior Cervical Vascular Complications
Vascular complications in the cervical spine can result from improper and excessive retraction or inadvertent laceration with a scalpel or other surgical instrument.41–66 The absolute incidence of vascular injuries from anterior cervical spinal surgery is not known, but it is less common than in anterior thoracolumbar surgery, approximated at 0.5%.67 Neo and colleague’s32 review of 5641 cases placed the incidence of vertebral artery injury at 0.14%. In a review of complications of anterior spinal surgery involving more than 500 cases, Tew and Mayfield19 reported no vascular injuries. Nor did Bertalanffy and Eggert38 in their review of 450 cases. Hohf reported four cases of carotid artery injury during anterior cervical fusion, three involving laceration and the other inadvertent laceration of the carotid artery.131 No permanent neurologic sequelae occurred from these vascular injuries, but all were repaired primarily. Although Phillips emphasized that the vessels of the carotid sheath were at “some risk” from the self-retaining retractor, he reported no cases of injury in his series.69 Careful placement of self-retaining blunt blades under the longus colli muscles medially and laterally and not against the carotid sheath is the safest method in anterior cervical surgery in prevention of carotid artery injury.
Rizzoli70 reported one case of vertebral artery laceration during anterior discectomy and fusion from a pituitary rongeur laterally in the neuroforamina. Typically, bleeding in this case was controlled by packing with oxidized regenerated cellulose. No central or peripheral neurologic complications or sequelae developed. Historically, Schweighofer and colleagues71 reported a single vertebral artery injury in 175 cases of interbody fusion. The artery was sacrificed and there were no adverse consequences for the patient. Golfinos and colleagues72 reported four cases of vertebral artery injuries in 2015 anterior cervical spine cases for a case series incidence of 0.3%. Martin and colleagues73 used a Cloward technique for anterior discectomy and fusion without any major vascular injuries in 167 degenerative cases. Microvascular issues in cervical spondylotic myelopathy were a significant factor in neurologic deterioration and/or progression.17,74,75 Multiple authors have written about the microvascular aspects of spinal cord injuries, both acute and chronic.76–80 Although classically a type of vascular disorder, this topic is more comprehensively covered in the section(s) on cervical degenerative disc disease (see Chapter 36). This type of vascular injury illustrates the consequences of ischemia on an end organ system.76–81 In reality, vertebral artery injuries occur almost exclusively compared with carotid artery injuries in anterior cervical surgery.
Exposure of the anterior cervical spine commonly uses an approach medial to the sternocleidomastoid muscle and carotid sheath (Smith-Robinson approach).29 Other approaches may dissect medial to the sternocleidomastoid muscle but lateral to the carotid sheath or lateral to both.20,82–84 These approaches risk direct injury (laceration) to the external and internal jugular veins. The external and internal jugular veins are of little clinical significance, except in regard to hemorrhage, and may increase the rate of upper extremity thrombosis.85,86 However, laceration of a large vein may be of significance if the patient is positioned in an extreme reverse Trendelenburg or sitting position because of the negative pressure created, which may result in an air embolism with disastrous sequelae.87 Zeidman and Ducker88 reviewed their experience with the posterior approach to cervical radiculopathy. In their 172 cases done in the sitting position, four patients had air embolisms without clinical sequelae.88 Lacerations of the carotid sheath vessels should be repaired if possible, although the internal jugular vein may be ligated if necessary.17 Kratimenos and Crookard89 presented 15 cases of far lateral exposure instead of a transoral approach to the base of the skull and foramen magnum. No vascular complications were reported, but the exposure required identification, protection, and retraction of the horizontal portion of the vertebral artery.89
The common carotid artery is at risk as one develops the dissection plane and during retraction for the deep anterior cervical spine exposure (Smith-Robinson approach). Identification of the carotid artery by palpation and gentle finger dissection helps to minimize injury of this major vessel. Proper placement of self-retaining retractors requires medial to lateral mobilization of the longus colli muscles along the anterolateral aspect of the vertebral bodies. Blunt-tipped blades of these self-retaining retractors must be under these muscles, and only enough tension should be applied to retract these muscles away from the vertebral and disc margins aiding in visualization of the vertebra and vertebral disc. An additional preventative measure during surgery has the anesthesiologist monitor the superficial temporal artery pulse for possible carotid artery occlusion secondary to excessive retraction.17,80,90 Pollard and Little91 documented a progressive drop in carotid blood flow as a consequence of self-retaining retractors. Ipsilateral carotid artery blood flow changed from 70% to 14% on average measured by duplex ultrasonic flow as the anterior cervical discectomy and fusion proceeded in 15 cases. The diminished blood flow remained laminar at all times and rapidly returned to baseline postoperatively. Younger patients showed a greater drop in blood flow compared with older, more atherosclerotic patients. Two cases of stroke from a presumed overzealous retraction of the right carotid artery using handheld Richardson retractors, resulting in a left hemiplegia, are known to the authors. One patient improved, but the other did not. Obviously, this complication is more likely in the older patient with atherosclerotic disease, requiring extensive exposure and dissection (e.g., multiple corpectomies).92 Unquestionably, compromise of carotid artery blood flow is highly likely to cause central nervous system ischemia (i.e., stroke).92–94
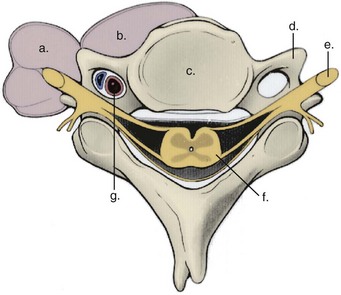
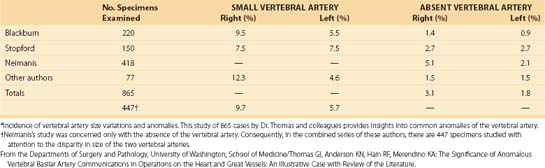
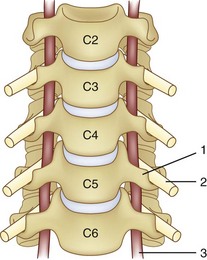
The vertebral artery and vein are at risk from both anterior and posterior exposures of the cervical spine (Fig. 96–2).2 This is a unique feature of the cervical vascular anatomy. The vertebral artery is the only artery integrated with the spinal column (intraosseous) classically passing anterior to posterior at C1-C2.95 In his detailed anatomic studies, Thomas and colleagues96 have presented the incidence of vertebral artery size, variations, and anomalies (Table 96–3). In their classic surgical treatise, Smith and colleagues61 suggested that the incidence of vertebral artery injury in anterior cervical surgery is less than 0.5%. Vertebral artery injuries result in either hemorrhage, vertebral basilar insufficiency stroke, or both.61,95,97 During anterior exposure, the vertebral arteries are at risk of injury by dissection lateral to the longus colli muscles, whereas during an anterior discectomy the lateral margins of the disc past the uncinate process are at risk (Fig. 96–3).61,98 Additionally, prevention of vertebral artery injuries laterally begins with careful dissection of the longus colli muscles. The anatomic relationship of the vertebral artery in the transverse foramina has been further studied by Ebraheim.99–102 The vertebral artery is tethered to the transverse foramina starting at C6 and proceeding to C2. This occurs by a fibroligamentous mesh connected to the adventitia of the vertebral artery, the vertebral body, and the upper edge of the transverse foraminal bony extension (Fig. 96–4). This is most likely to have an impact in cervical trauma but has implications if anterior vertebral artery laceration repair is attempted, especially in mobilizing and controlling the distal and proximal ends of the artery.
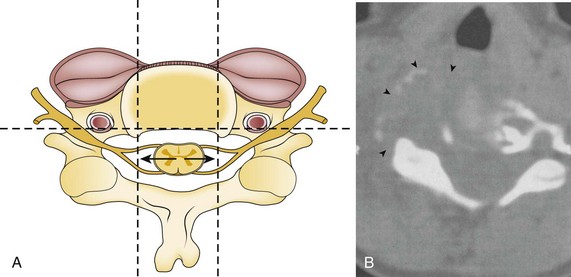
Coagulation of the medial edge of the muscles and the use of a blunt periosteal elevator to mobilize the muscles laterally are recommended. The periosteal elevator should never be directed posteriorly. More likely, the vertebral artery is injured with excess lateral dissection in the intervertebral space past the uncinate processes (C3-C6), especially in case of corpectomy and tumor (see Fig. 96–3). Dissection and/or bony resection should be medial to the uncinate process sparing this medial border of the transverse foramen unless otherwise necessary (e.g., tumor). To avoid injury to the vertebral artery injury in corpectomy, most authors recommend a midline radiograph or a computed tomography (CT) scan for any abnormalities of the vertebral artery in the transverse foramina, also knowing that the longest colli muscle margin medially is the most consistent marker for the lateral extent of bony dissection.
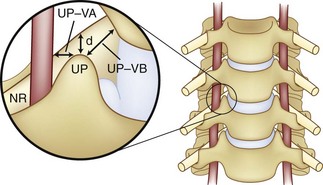
If foraminotomy is required, it is done so with 1 mm Kerrison punch undercutting the inferior aspect of the uncinate process, where the vertebral artery is unlikely to be injured as long as only the posterior aspect was undercut. Use of the operative microscope and microinstruments within the disc space has been advocated to help decrease the risk of vertebral artery injury at the C3-C6 levels.35,103 In the 1215 case series of Golfinos and colleagues,72 four vertebral artery injuries were caused by decompression laterally in two, screw tapping, and soft tissue retraction (one). Lu and Ebraheim have studied the vertebral artery anatomy, demonstrating that it is at risk for injury lateral to the uncinate process from C3-C6 as the vertebral artery angles increase medially by 4.3 ± 2.6 degrees from caudal to cephalad (Fig. 96–5).67,99,104 The vertebral artery in essence converges medially from its origin at the C6 transverse foramina. The vertebral veins accompanying the vertebral arteries are located medially to the artery and are the first vessels “at risk.” The use of the high-speed burr lateral to the uncinate process was implicated in a majority of reviewed cases of vertebral artery injury during anterior corpectomy and foraminotomy (Fig. 96–6).54,67
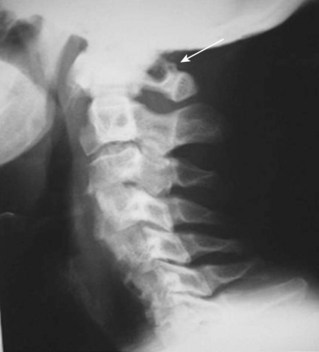
Anomalies of the vertebral artery have been reported to directly compress the cervical spinal cord and nerve roots causing myelopathy and radiculopathy (see Table 96–3).54,105 The vessel was either elongated, reduced in size, or tortuous. This type of enlargement as shown by Slover and colleagues,106 gradually erodes bone, compresses the neural elements, and risks injury if discectomy or corpectomy is demanded at the anomaly level. Surgical resection and reconstruction decompression and repairing the pathologic vertebral artery were successfully employed in these uncommon cases.106 A vertebral artery injury from a C2 compression screw used to treat a type II odontoid fracture was reported by Daentzer and colleagues.107 This may occur from the guidewire, especially during insertion, or may simply be a screw that is too long (Fig. 96–7). Type II C2 fractures are increasingly treated with cannulated 3.5- or 4.0-mm partially threaded screws. Obviously, a guidewire can be as “dangerous” as the overinserted screw relative to the vertebral artery. Dickerman and colleagues108 reported a case of anterior cervical corpectomies C6 in which a postoperative stroke occurred secondary to an injury of the left vertebral artery.
The vertebral artery and cervical spine trauma has been increasingly recognized and studied (see Chapter 82). Although not directly related to our topics, this high-energy injury provides insights into the consequences and sequelae of unilateral and/or bilateral vertebral artery injuries. They are most often associated with a cervical fracture dislocation or rotatory subluxations. These subluxations occur at about 0.05% incidence including all trauma and 70% of cervical spine trauma. The treatment is often nonoperative. Benign neglect is certainly reasonable if there are no neurologic deficits, but spinal alignment and stability should be rapidly restored. Fassett and colleagues109 found a vascular injury rate with neurologic sequelae from 0% to 24%. The diagnosis is established by cervical angiography or secondarily by magnetic resonance angiography (MRA), both possibly used as a noninvasive alternative treatment; is widely variable; and is not defined by Level I and II evidence-based research. Hoit and colleagues110 studied blunt craniovertebral trauma in 69 patients. The most common injury was a basal skull fracture with neck hyperextension. The carotid artery was also monitored and quantified. Carotid artery injuries are more likely to lead to a cerebral vascular accident and are highly associated with facial fractures. Neural sequelae and/or vascular sequelae of the vertebral artery can result in a Wallenberg or lateral medullary syndrome with ischemic defects, cerebrovascular accident (CVA), and arterial hemorrhage. This is also called the “bow hunter syndrome,” referring to a stroke produced by vertebral artery occlusion.111 Of the 69 cases observed by Fassett,109 20 had vascular blunt injuries where 3 carotid and 15 vertebral arteries were involved. The pathophysiology of the carotid artery and vertebral artery injury showed intramural dissection (8 out of 28), pseudoaneurysm (6 out of 28), and occlusion (5 out of 28). Eight injuries occurred intracranially and had the worst prognosis. There were 20 in the cervical region alone. Additionally, Taneichi and colleague’s112 review of cervical spine trauma found a 7.2% rate of vertebral artery injury. In this series, the primary cause of injury was a cervical fracture dislocation from C2 to C7. Three of the 11 injuries restored flow without treatment, and occlusion was rare, but it was much more common to see intramural dissection, especially with the fracture dislocation. All of their 11 cases, one bilaterally, were asymptomatic.
Undoubtedly, blunt head and neck trauma risks the vertebral artery, especially if there is any translation or rotation of the spinal segments of C2 to C6. Vascular vessel lesions may be spasmodic, dissections, lacerations, and occlusions. However, most are prevented by rapid reduction of the cervical spine. If there is a noninvasive standard for studying the artery, the MRA is probably the best choice. Asymptomatic occlusion or other injuries are treated with observation. If the artery is occluded and symptomatic, it can be restored either through open surgery or more likely through the newer endovascular techniques for opening an occluded or damaged vessel.113 Clinical results in symptomatic patients depend on the rate at which the cervical spine can be reduced and the time of ischemia. However, endovascular repair with coated stents placed in the injured vertebral artery are superior to classical open vascular repair.
DiFabio114 performed a large inclusive series in which he reviewed 116 articles of vertebral artery injury. The most common injury was an arterial dissection followed by a basilar skull injury followed by a lateral medullary syndrome. Two percent of vertebral arteries were injured from manipulation during physical therapy. Overall morbidity was 10%. A warning or “soft” sign on physical examination is to have the patient maximally externally rotate to the right and left for 30 seconds. This is a preoperative predictive sign of spinal cord foraminal patency. If the patient does not develop symptoms, have the patient thrust the neck forward and repeat the rotation for 30 seconds. If no symptoms develop with these two maneuvers, then, in theory, the vertebral artery is patent and proximal flow is normal. A paradigm shift recently has occurred in repair of the vertebral artery from primary suture to endovascular repair. This technique used coils or covered stents and can now be inserted into the vessel to avoid complete occlusion while stopping any bleeding while occluding the vessel wall defect. This would be especially important to the left side vertebral artery, which is thought to be most frequently dominant.96
The inferior and superior thyroid arteries, between the sternocleidomastoid muscle and carotid sheath, are also at risk to injury in anterior approaches. Avoiding injury to these vessels is important not only for the sake of bleeding but also because each has a significant nerve accompanying it—the superior laryngeal nerve and recurrent laryngeal nerve, respectively. If necessary, the vessels themselves may be divided and ligated for additional exposure.35,43 Jenis and LeClair115 reported an unusual case of an inferior thyroid artery pseudoaneurysm 9 days after a routine anterior cervical discectomy and fusion. Selective endovascular embolization without revision surgery was successful in correcting this condition.115
The anterior spinal artery system is essentially an independent vascular system in the upper cervical segments and depends on contributions from radicular arteries in the middle and lower segments. The principle segmental vessel enters on the left at C6 branching from the deep cervical artery.116 The anterior spinal artery is at risk of compression in cervical spondylosis by anterior disc or osteophyte. If appropriate for the case, preservation of the posterior longitudinal ligament protects against epidural bleeding and possible injury of the anterior spinal artery. The avoidance of unipolar coagulation around the posterior longitudinal ligament or the dura is to be emphasized.38,74 Inadvertent coagulation or disruption of the anterior spinal artery will likely result in quadriplegia or quadriparesis from ischemia of the anterior and lateral cervical spinal cord.79,117,118 Regarding complications related to microvascular disease, myeloradiculopathy can cause dysfunction in the esophagus even before surgery.
Iatrogenic arteriovenous fistula between the vertebral artery and the surrounding venous plexus is a rare complication of anterior cervical discectomy.119 It may complicate anterior cervical discectomy at or above the C5-C6 level because the vertebral artery runs immediately lateral to the disc space beginning at this level. The presence of a cervical bruit following surgery may suggest a fistulous communication and necessitates angiographic investigation. Minimally invasive endovascular occlusion of the fistula with contemporary interventional techniques is the therapeutic procedure of choice.38 Cosgrove and Théron119 reported a patient who presented with an audible bruit 2 months after successful anterior cervical discectomy and fusion. Revision surgery was not necessary. The author is aware of two unreported cases of vertebral artery injury during anterior cervical disc surgery. Both resulted in no permanent injury, although one case had a pseudoaneurysm detected by postoperative magnetic resonance imaging (MRI) and MRA and ultimately was treated with microscopic reconstruction of the vertebral artery.17
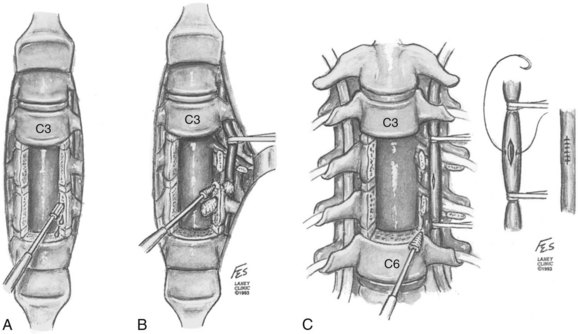
Treatment of the vascular injuries during anterior cervical spine exposure involves recognition, control of bleeding, and repair, ligation, or endovascular stents when indicated. Although primary repair of the vertebral artery and common carotid artery is desirable, endovascular techniques are becoming the standard of care. If the vertebral artery is lacerated during the anterior dissection, the bleeding point must be tamponaded and the dissection carried laterally by mobilizing or dividing the longus colli muscles. The costotransverse lamellae and fibroligamentous attachments can be removed with a high-speed, diamond burr or Kerrison rongeurs to allow adequate exposure for primary vascular repair (Fig. 96–8A).120 If primary repair is chosen, the artery should first be exposed proximal to the laceration and then the loops should be used for occlusion (Fig. 96–8B).120 The nerve roots should be identified and protected. Once the artery is exposed and controlled, the laceration can be repaired, if possible, with 7-0 polypropylene interrupted sutures (Fig. 96–8C).120 The repair will require the assistance of an experienced vascular surgeon, and spinal stability should be assured after this additional required exposure. If temporary, cross clamping of the injured vertebral artery required in the repair in effect mimics a ligation and vertebrobasilar ischemia or infarct still may occur. Repair of vertebral artery injury is supported by studies by Golfinos, Pfeifer, and colleagues.72,93,120 Others have ligated the lacerated vertebral artery during an anterior approach to the cervical spine.2,38,95,104,121 This presumed a planned resection, which is rarely a realistic fact. If the vertebral artery has to be sacrificed, ideally but not practically it should first be studied by intraoperative angiography to ensure that the anatomy of the patient’s vertebral system is likely to permit such a maneuver.82,95,104 The ability of the patient to tolerate unilateral occlusion of the vertebral artery with sequelae is supported by a study of nine patients with traumatic occlusion of the vertebral artery. Only two developed neurologic deficits, both of which were transient.122 The presence or absence of neurologic deficit depends in part on the collateralization of the vertebral artery of the circle of Willis and the status of the opposite vertebral and basilar arteries (see Table 96–3).2,38,43 However, Smith and colleagues61 reported three out of seven cases of vertebral artery ligation developed symptomatic vertebrobasilar ischemic signs and symptoms. These symptoms include syncope, drop attacks, dizziness, nystagmus, and the Wallenberg syndrome. Smith and colleagues61 recommended primary repair if it could be accomplished, but repairs were not done in their retrospective series. These experienced surgeons could only (1) tamponade (30%); expose and electrocoagulate (10%); ligate transosseously (20%); or (2) open hemostatically clip (10%) in their reported 10 cases (Fig. 96–9). Further controversy as to whether to ligate or repair a vertebral artery laceration or tear is found in the cervical spine trauma literature. Thomas and colleagues96 presented a classic anatomic study that occlusion of the vertebral artery related to the incidence of brainstem ischemia or infarct was 3.1% with left-sided occlusion compared with 1.8% with right-sided disruption of flow (see Table 96–3). Conventional arteriography and, more recently, MRA demonstrate frequent vertebral artery flow abnormalities in injuries such as unilateral and bilateral facet fracture dislocations.49,77,78,123–125 In most cases, thrombosis occurs with or without recanalization.122 Despite being common sequelae, the injuries rarely cause serious irreversible vertebrobasilar ischemia or infarction of the brainstem and cerebellum.44 Reid and colleagues126 found 43 cases of vertebral artery injury from head and neck trauma. There were no cases of vertebral basilar insufficiency or stroke. However, there was a 4.7% morbidity due to hemorrhage. The minimally invasive endovascular repair is becoming the procedure of choice in symptomatic or hemorrhagic vertebral artery (see also Chapter 82).
A specific vascular complication resulting from local hemorrhage with potentially lethal consequences is a wound hematoma.127,128 This cannot be prevented by intraoperative drain placement.129 Wound hematomas in the neck represent a spectrum of clinical presentations from small local swellings to deeper collection compressing the trachea or spinal cord. These can present within hours to days following anterior cervical surgery. In cases with rapid deterioration and/or an expanding hematoma, immediate decompression of the neural elements or airway may be required to open the wound as expediently and sterilely as possible. Further discussion is given later in “Subdural and Epidural Hematoma.”
Vascular complications of cervical total disc arthroplasty are similar to those discussed for anterior cervical discectomy and fusion (see Chapter 43). Vascular complications are rarely associated with extensive discectomy because the uncinate processes are preserved. Suffice it to say, as long as disc preparation before implantation does not violate the uncovertebral joint, injury to the vertebral artery will remain rare. All of these potential vascular complications apply for the Smith-Robinson exposure. Intuitively, it is unlikely that a constrained total disc arthroplasty in the neck will fail by extrusion but more likely by bone fracture settling or polyethylene wear.130
Posterior Cervical Vascular Complications
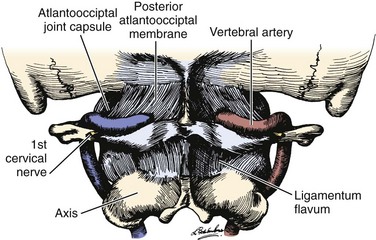
The vertebral artery and venous plexus are the only major vessels that course posterior to the lamina in the spinal column (Fig. 96–10). These vessels are at risk of injury in the upper cervical spine or during posterior fossa surgery.35,95 Fortunately, the incidence of posterior vertebral artery injury is rare. Rizzoli70 reported three cases of posterior vertebral artery injury, all of which were secondary to laceration from the bovie device; two were ligated without neurologic deficits, and the other was repaired primarily. Hohf131 reported one case of posterior vertebral artery injury during posterior cervical spinal surgery, but the details of the injury were not given. The author is aware of two cases of posterior vertebral artery injury during exposure of C1 for an occipital cervical fusion. Both of these cases resulted in a liter of blood loss from vigorous subperiosteal dissection with a periosteal elevator. Both were managed by packing to control bleeding with absorbable gelatin sponge or oxidized regenerated cellulose. Fortunately, no neurologic sequelae developed in either case.17 Cranial base surgery continues to offer significant challenges and limitations owing to the variations of this region’s cerebrovascular anatomy and physiology (see Table 96–3).132
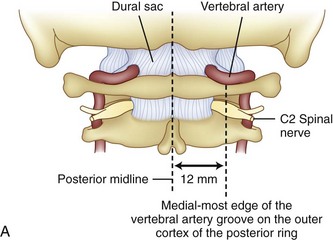
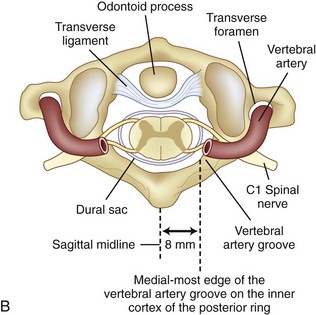
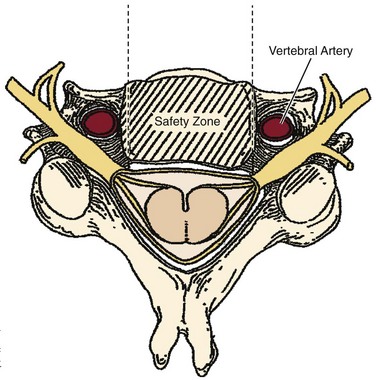
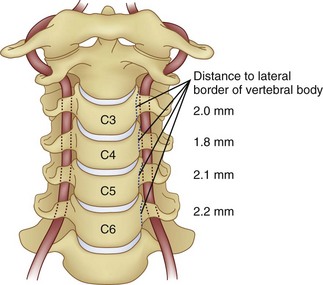
Prevention of vertebral vascular injuries during posterior exposure of the upper cervical spine starts with identifying the posterior tubercle of the atlas and limiting lateral dissection to less than 1 cm (8 to 12 mm) along the cephalad edge of C1 and less than 1.5 cm (12 to 23 mm) along the caudal C1 border (Fig. 96–11).17,95,104 These limits may need to be adjusted to take into account smaller individuals or pediatric patients. The vertebral artery is vulnerable because it passes between the C1 and C2 transverse foramina laterally. Here, lateral dissection along the caudal border of C1 should stop when one encounters the dorsal ramus of C2. Injury to the vertebral vessel should be controlled initially by packing and administering local antithrombotic agents. If possible and technically feasible, a posterior vertebral artery injury should be repaired for similar reason for repair in the transverse foramina (i.e., to preserve brainstem and cerebellar blood flow).132
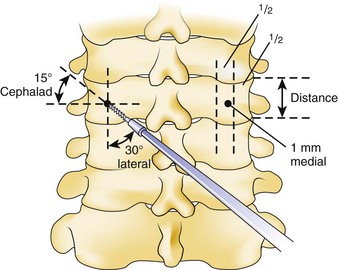
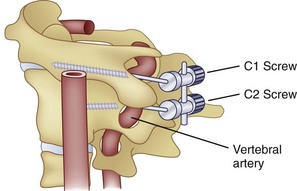
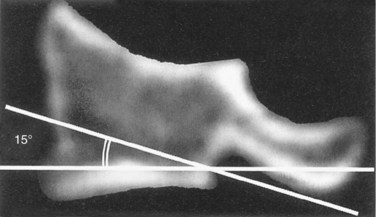
Similar to anterior lumbar surgeries, instrumentation of the upper posterior cervical spine has seen an exponential increase. Consequently, operating from the occiput to C2 increases the risk of injury to the vertebral artery, carotid artery (CA), and hypoglossal or cranial nerve XI depending on the technique. Malpositioning of the lateral mass screws may injure the vertebral artery within the transverse foramen (Fig. 96–12).2,20,102,133 As this technology is becoming established as a standard of care, complication rates of C1-C2 transarticular screws and C1 lateral mass and C2 pars screws are increasingly reported (see Fig. 96–12). C1-C2 transarticular screws risk the vertebral artery, carotid artery, and hypoglossal (CNX11th) nerve anteriorly in the C2 transverse foramina, making preoperative CT scanning mandatory to prevent inadvertent injury as screw insertion progresses across the C1-C2 facet (Fig. 96–13).97,134 Anomalies and anatomic variations are common (10%) among all cases in the geometry of the foramen, as well as its position with the vertebral artery in the C1 vertebral body (CV6). C1 lateral mass screws risk the vertebral artery posteriorly and laterally, requiring meticulous technique for mobilization and protection.18,135 Prevention includes knowledge of the bony landmarks, choosing appropriate screw lengths, and reviewing angles of insertion (Fig. 96–14). Wellman and colleagues,138 in a consecutive series of 43 patients, reported no vertebral artery injuries from lateral mass plating using AME Hard Universal instrumentation (UBP; American Medical Electronics, Richardson, Texas). It is noteworthy that the females average a 14- × 3.5-mm screw compared with a 16- × 3.5-size for males.83 The vertebral artery and veins are directly anterior or slightly lateral (about 6 degrees at C6) at the middle one third of the lateral mass. A lateral mass screw insertion technique, starting near the center of the mass and directed about 10 to 15 degrees lateral and 30 to 35 degrees cephalad, is best to avoid the vertebral artery.
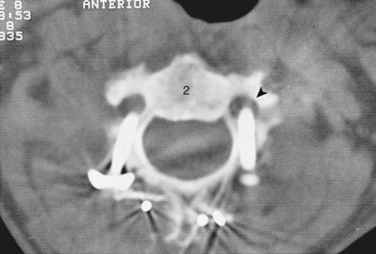
FIGURE 96–12 Malpositioned C2 screw violating the vertebral artery, fortunately without ill effects or vascular sequelae.
The Magerl technique, which goes more or less straight down from a central lateral mass, is more likely to impale a nerve or the vertebral artery. Thus this technique should be avoided. Cervical pedicle screws may be placed more safely at C6 or C7 in reference to the vertebral artery. The screws are started about 2 to 3 mm inferior to the superior articular facet and just lateral to the midline of the lateral mass angling medially 35 degrees (C6) and 30 degrees (C7). These projections pass anterior to the vertebral artery at C6 (see Fig. 96–14). The C1-C2 technique, which is least likely to risk injury to the anterior VA, is the modified technique of C1 lateral mass screws and C2 intralaminar crossed screw fixation described by Harms and others.136 In about 15.5% of patients, a false bony bridge called the ponticulus ponticus covers the vertebral artery as it passes onto the posterior C1 ring. This is easily noted on lateral cervical plain films (Fig. 96–15). Young and colleagues137 emphasized the importance of recognizing this common anomaly to avoid injury to the VA by dissection or if C1 transosseous screw fixation is considered.
Placement of anterior C2 screws is routinely used to stabilize type II or high type III odontoid fractures. Remember that C1 and C2 injuries anteriorly or posteriorly should be reduced before attempting fixation or there will be a much higher risk of injury to the cord or vertebral or interior carotid artery. Vascular risks are derived from either the guidewire or screw tip, which may impale the internal carotid artery or the vertebral artery (Fig. 96–16). To avoid this, radiologic control is used to minimize penetration of the tip of the odontoid at initial positioning, whether you use one or two screws and guidewires. C2 anterior screw placement should be done with live fluoroscopy by hand and without power reaming and tapping. Once positioned, make sure the reamer or screw cannula is free of bone so that inadvertent advancement of the guidewire does not occur. This procedure increases fluoroscopy time, but it is essential to carefully monitor the tapping position, as well as the insertion over the guidewire, to make sure that proximal migration does not occur. Some surgeons employ three-dimensional (3D) fluoroscopy or CT image guidance in such cases to minimize radiation while maintaining safety by imaging guidance (see Fig. 96–7).
Vertebral artery injury posteriorly is often injured by lateral mass screws either from the drill, screw, or probe penetration. All the techniques that start near or just inferior to the midline and angle laterally and cephalad 15 to 30 degrees and use screws that are 14 to 16 mm are unlikely to injure the vein (vertebral artery or veins).102,138 As with anterior C2 screw placement, drilling should be done by hand and not power. Also, caution should be used with the depth gauge taking care not to plunge and possibly grab the vertebral artery with the curved end of the depth gauge. Neo and colleagues32 clearly identified the Magerl technique as most likely to injure the vertebral artery in the lateral mass technique with an incidence of 1.4% in his survey series of 5,641 cases. The relationship of the vertebral artery to C1-C2 and its location to the triangular window of the pedicle of C1 is emphasized by Ebraheim and colleagues99,100–102 (Figs. 96-16 to 96-18; see Fig. 96–11A). Primary clinical challenges are to identify the C2 root, identify the vertebral artery, and identify and control the venous plexus, which sometimes covers and projects inferiorly between the vertebral artery and the posterior C2 dorsal ramus.
C1 fixation was complicated in one series by high-riding vertebral artery, thinning, or abnormality of the C2 pedicle, where anomalies of the artery are common in the vertebral artery (see Figs. 96-11A and 96-17). Typically 8 mm from the C1 tubercle, the vertebral artery penetrates the ligamentum flavum passing over the cephalad margin of the C1, posterior ring. The level of the C1 lateral mass screw insertion at the inferior border of the C1 arch is typically 12 to 14 mm from the midline. The ponticulus ponticus is a harbinger for injury to the vertebral artery along the cephalad margin, especially for C1 lateral mass screws, if unrecognized (see Fig. 96–15).
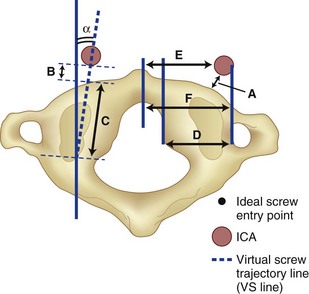
C1 lateral mass fixation has proven safe, stable, and effective on the basis of the window of the lateral mass with the C1 arch and the vertebral artery superior and the C2 dorsal root ganglion inferior. This “window” is covered by a plexus of veins (differentiated from vertebral artery), which needs to be coagulated and controlled. Removal of the inferior edge of the C1 arch often assists the placement of the C1 lateral mass or pedicle screw. The medial-most extension of the vertebral artery cephalad to the C1 ring is usually about 8 mm from the C1 tubercle but about 4 more mm lateral and caudal to the C1 ring, which is the optimal starting point for a C1 lateral mass screw. Image guidance systems like 30 fluoroscopic or CT-based guidance systems are becoming vital and increase safety in this point of the C1 lateral mass screw positioning.139 The vertebral artery anomalies are high in relation to the vertebral artery to transverse foramina and bone and to the anastomosis at the bone and skull. Thomas and colleagues96 found an absence of the vertebral artery on the right in 3.1% and 1.8% on the left. They reported several cases of narrowing of the vertebral artery (5.7% on the left and 9.7% on the right). Additionally, the left vertebral artery is most often dominant. Also, Thomas and colleagues96 found two cases of vertebral artery proceeding directly to the posterior internal cerebellar artery, one on the right and one on the left. These anomalies can and do affect C1 lateral mass screw fixation along with the ponticulus ponticus (see Fig. 96–15). A preoperative CT with reconstruct is mandatory, as is the upper cervical case, where screws are placed so as to identify the vertebral artery and its foramen. Numerous minor and major variation occur, some of which preclude screw placement on one side. If injury of a vertebral artery occurs, it is treated by reinsertion of the screw or in some cases packing the hole with collagen cellulose strips and covering the hole with sterile bone wax.
Thoracic Spine
Anterior Thoracic Vascular Complications
Anterior exposure to the thoracic spine is well established with ever-increasing applications.4,17,45,88,140–161 Thoracoscopy is now routine for the treatment of a variety of disorders, primarily scoliosis release, fusion, or instrumentation. Oskouian and Johnson11 retrospectively reported a 5.8% incidence of vascular complications in 207 open anterior thoracolumbar surgeries with mortality rate of 1%. They reported a 3.4% incidence of direct vascular injuries of the segmental or intercostal vessels. Most had a delayed onset of sequelae, and one death occurred as a result. Potential vascular-related complications involve injury to the major vessels and their branches and the consequences of the interruption of the blood supply to the thoracic spinal cord (Fig. 96–19). The incidence of paraplegia secondary to unilateral vascular interruption of the thoracic segmental vessels is essentially nil, in primary or unoperated cases. Bilateral disruption of the segmental blood supply, as in aortic surgery or dissecting aortic aneurysms, is not as forgiving.21,81,162–168 The thoracic and vascular literature is replete with reports of the difficulties with bilateral disruption of the blood flow to the thoracic cord during complex thoracic and thoracolumbar aneurysm reconstructive surgery. Recently, the risk of paraplegia and paraparesis in cardiothoracic surgeon has been reduced by (1) limiting cross clamping times,21,162 (2) avoiding hypotension (i.e., mean arterial blood pressure [MABP] < 60 mm Hg),21,162 (3) cerebrospinal fluid (CSF) drainage,163,165 and (4) distal aortic perfusion by cardiopulmonary bypass.21,163 CSF drainage or cooling is proven to decompress the intradural space and improve blood flow to the cord via the artery of Adamkiewicz and other segmentals.163,165 Parke and colleagues12 have shown that smooth muscle of the tunica media of the lower anterior spinal artery may further risk blood flow to the cord if constricted by autoregulation after aortic cross clamping. Other procedures associated with spinal cord infarction include bilateral sympathectomies16; open thoracoscopy with lobectomy169; open anterior surgery for tuberculosis in which bilateral vascular ligation or disruption occurs166; revision anterior scoliosis surgery, especially if approaching the previously undissected side (i.e., right side) of the anterolateral spine or having a significant kyphotic component167,170; and rarely a routine spinal test today, angiography, and MRA using the thoracic or abdominal aorta.44,72,164 Theoretically, the thoracolumbar spinal cord tolerates transient and permanent unilateral segmental blood flow disruption. However, bilateral segmental disruption, transient or permanent, is much more likely to cause spinal cord ischemia and thus paraparesis or paraplegia.
The exact incidence of spinal cord infarction and paraplegia due to vascular occlusion is not known. Morbidity and mortality statistics from the Scoliosis Research Society (1975) described an incidence of 1% paraplegia due to indirect vascular compromise (distraction instrumentation) in more than 10,000 deformity cases.95,171,172 Paraplegia rates are higher in adult patients and those treated with posterior instrumentation and fusion for severe scoliosis, kyphosis, kyphoscoliosis, or congenital scoliosis and kyphosis.173,174 Open, single-stage, same-day vascular disruptions leading to paraplegia have been reported in 6% of one-stage circumferential spinal osteotomies.36 Of the five reported cases, four were permanent and one recovered completely. In this series of circumferential osteotomies, there were 55 adults with scoliosis and all cases resulting in paraplegia were at the T5-T9 level. Hodgson reported one case of vascular-induced paraplegia in more than 400 cases of anterior spinal surgery for tuberculosis.166 Riseborough,148 Micheli and Hall,174 Dwyer and Schafer,142 and Winter and colleagues151 reported a cumulative total of more than 3000 extensive open anterior discectomies or partial corpectomies with unilateral ligation of thoracic segmental arteries without any cases of paraplegia. It is important to understand that unilateral ligation of thoracolumbar segmental arteries applies to primary cases and occurs over the convexity of the deformity. Certain complex revision surgeries, however, may mimic bilateral ligation.151 Cases in which there is significant kyphosis or kyphoscoliosis, especially with multiple posterior procedures or prior surgery, may have paraplegia with unilateral ligation of “key” segmental arteries. Winter and colleagues151 discuss a so-called “cord at risk” advocating spinal cord monitoring up to 20 minutes after ligation or clamping of a key segmental (Artery of Adamkiewicz). These authors describe a case of revision anterior scoliosis surgery in which the convexity was approached after prior anterior surgery from the concavity.151 The authors clamped a segmental and observed loss of somatosensory evoked potentials (SSEPs). The spinal cord monitoring changes were reversible and mandated that the anterior discectomy be performed between the segmental vessel, thereby sparing spinal cord blood flow. This circumstance illustrates that bilateral thoracolumbar segmental vessels are associated with a small but definite risk of spinal cord infarction and permanent neurologic deficits. Avoiding actual or indirect bilateral segmental vessel injuries should be avoided from T9-L2 on the left.
The transthoracic approach to the anterior thoracic spine usually requires mobilization of segmental arteries and veins over several vertebral levels.98,142,148,151,174,175 A right-sided approach is recommended to avoid the pericardial structures and theoretically avoid the artery of Adamkiewicz. Spinal cord monitoring is now considered routine, especially motor evoked potentials (MEPs). MEPs are the most precise method to monitor the spinal cord (anterior spinal cord) compared with SSEPs, which test primarily the dorsal columns of the spinal cord.176 In cases of deformity, however, it is most advantageous to approach the convexity of the deformity, regardless of the side involved. To gain access to the thoracic segmental and intercostal arteries, the parietal pleura must be divided.177 This dissection begins over the disc space, where there are no vessels. The individual vessels are identified and isolated with a right-angle hemostat and ligated; occlude with vascular clamps, or spared.178 About 1 centimeter of segmental vessel is maintained from the thoracic aorta or vena cava to avoid cutting this vessel too close to the aorta or vena cava, thereby creating a hole in the side of the vessel. Lazorthes and colleagues179 have shown that better collateralization and anastomotic substitution can occur as these vessels are ligated or disrupted closer to the major vessels. Inadvertent laceration or avulsion of the segmental vessels from the aorta or cava can result in rapid voluminous bleeding.17 Should this occur, bleeding should be controlled by direct pressure proximal and distal to the defect. Repair of the aorta or vena cava should be assisted by a surgeon experienced in vascular surgery. Mack and colleagues143,149,180,181 reported 100 cases of video-assisted thoracoscopy (VAT) for a variety of thoracic spine conditions. A recent European meta-analysis found no vascular complications or paralyses associated with VAT.182 A singular case of profound epidural bleeding occurred, but no incident of segmental artery, aorta, or azygos vein injury was noted.104 Other studies of thoracoscopy have validated the usefulness and safety, especially relative to the vascular structures.68,146,149,183,184
Faro and colleagues in a thoracic spine calf model monitored thoracic vertebral body screws after placement in relation to the aorta at T6 to T11. This was an anterior thoracic instrumentation model. There were a number of penetrations of the vertebrae into the aorta. These penetrations at T6 to T11 were followed at 3, 6, and 12 months. Postmortem histology showed thinning of the aorta in 52%. Sixty percent had scarring with impregnation with a trend of greater impregnation causing larger amounts of scarring. In 96% of all the cases in which there was some screw contact, the aorta showed some cicatrix. The authors concluded that this histopathology was worrisome for late development of complications if the screw tip approached or entered the thoracic aorta in calves.185
The relative importance of individual blood vessels that supply the spinal cord is debated.94 In 1939 Suh and Alexander noted that “much of what has been written in the past 40 years about the circulation of the spinal cord is either inaccurate or incomplete.”81 In the same year, Bolton determined that the segmental vessels that supply the spinal cord are indeed end arteries and there are no anastomoses between the capillary beds.43 Individually, vessels that travel with the spinal nerve into the spinal canal do not seem to be important, as evidenced by Dwyer’s ligation of 3 to 16 ipsilateral segmental arteries in a single patient without neurologic loss.142 Great attention was given in the past to the artery of Adamkiewicz (arterial radiculitis or arterial magna),which is the largest of the feeders to the thoracolumbar spinal cord (Fig. 96–20).48,123,186,187 It occurs on the left side in 80% of patients between T7 and L4 with the greatest incidence (92%) between T8 and L1.80,117,187,188 It usually enters the spinal canal with at least one additional feeder. The location of the artery of Adamkiewicz has further been confirmed on the left from T8-L1 by helical CT scans and MRA, as well as more contemporary spinal cord angiographic techniques.167,187 In 1970 DiChiro and colleagues123 noted that this artery could be tied in Rhesus monkeys without complications, but paraplegia would follow when the anterior spinal artery (arterial median longitudinal artery) was simultaneously ligated. Further evidence of the importance of bilateral disruption of this segmental vessel is indicated by the high incidence of paraplegia in thoracic aortic surgery involving cross clamping of the thoracic aorta or in cases where dissecting aneurysms occlude the segmental vessels bilaterally.21,81,162,164–167,189,190 However, paraparesis or paraplegia as a result of unilateral ligation of thoracic, thoracolumbar, or lumbar segmental vessel (left or right) is rare.155 Bridwell and colleagues173 reported two cases in which there was unilateral ligation from T10 to T2 on the left, but these cases were complicated. One was a 34-year-old patient who had had kyphotic corrective surgery 6 years ago with an anterior and posterior treatment for kyphoscoliosis. Subsequently, he underwent another two-stage posterior repair of nonunion followed by an anterior left-sided T10 to T5 ligation of vessels with spinal cord monitoring a week later. During the second-stage surgery, the MEPs disappeared and the patient had a permanent anterior cord syndrome postoperatively. The other case was an 85-year-old male who had four prior lumbar decompressions and fusions, and 3 years before his presentation he had an L1 to L2 fusion. His diagnosis was flat back syndrome, and thus he underwent a posterior pedicle subtraction osteotomy at L1. Four months afterwards, he returned and underwent a revision anterior T10 to L2 left-sided fusion. On the second postoperative day he became a paraplegic. This delayed-onset paraplegia was attributed to slow occlusion of the artery of Adamkiewicz.155 Dr. Winter has always recommended that to avoid injury to the artery of Adamkiewicz to ligate the vessel unilaterally, it should be done in the midbody position and on the convexity of the curve. He also recommends, along with Leung, intraoperative monitoring, especially MEP or a Stagnora wake-up test if no monitoring is available or the spinal cord monitoring is felt to be inconsistent.191
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 to 2 cm from the midline.
to 2 cm from the midline.