CHAPTER 86 Infections of the Spine
Historic Perspective
The first recorded descriptions of spine infections were those in the Hippocratic texts on tuberculous spondylitis written between the 4th century bc and the 1st century ad. Sir Percival Pott’s description of paralysis in association with tuberculosis of the spine in the 18th century led to the eponym “Pott paraplegia.” His frustration with the inadequate treatment options available to him was shared by physicians for another 150 years: “To attend to a distemper from its beginning through a long and painful course, to its last fatal period, without even the hope of being able to do anything which shall be really serviceable, is of all tasks the most unpleasant.”1
Before the advent of antimicrobial therapy, the treatment of tuberculosis of the spine was based on bed rest, often in a plaster cast, with attention to diet and exposure to fresh air and sunlight. Laminectomy was the mainstay of surgical treatment in the late 1800s and the early part of this century but was later condemned by Seddon and others because it did not address the anterior disease and led to further instability.2 In 1911 Hibbs3 and Albee4 independently described the use of posterior fusion to hasten the recovery. The idea evolved from the demonstration that ankylosis of peripheral joints led to remission of local disease. Unfortunately, posterior fusion did not prevent progressive kyphosis or address the lesion that was causing paralysis, and the technique was later abandoned. The mortality rate for children treated by these various techniques was 40%.5 In 1894 Menard described a series of patients with Pott paraplegia successfully treated with decompression by costotransversectomy.6 The technique fell into disfavor because of a high rate of secondary infection, and it did not gain acceptance until Girdlestone reintroduced it in 1931 with aseptic technique.7
Ito and colleagues8 described the anterior approach to the lumbar spine in 1934 and demonstrated that it provided wider exposure and allowed more radical débridement and fusion. Hodgson and colleagues338,342 popularized the anterior approach for the management of tuberculosis of the spine and stressed radical excision and strut-graft fusion to prevent kyphosis and late-onset paraplegia.
Antituberculous chemotherapy became available in 1945 and was found to be capable of curing the disease even without surgery.9–13 Faced with a number of widely divergent regimens for the treatment of the disease, a group of investigators formed the British Medical Research Council Working Party on Tuberculosis of the Spine. This group set out to perform a number of large-scale, controlled prospective trials of treatment methods. These studies, as well as others to be described later, helped to determine the current treatment recommendations for this disease.
The first recorded description of a pyogenic spine infection was by Lannelongue in 1897.14 Although pyogenic spine infections differ in many ways from tuberculous spondylitis, the surgical treatment of the former has been influenced a great deal by the developments in the management of tuberculosis. The introduction of penicillin and streptomycin revolutionized the treatment of all spine infections. As more powerful antimicrobial agents were developed and combinations and dosages were refined, the relative effectiveness of surgical treatment decreased.
Pyogenic Infections
Vertebral Osteomyelitis
Epidemiology
Although the incidence of tuberculous spondylitis has decreased dramatically in recent years, the incidence of pyogenic vertebral osteomyelitis appears to have increased.15,16 Various reports have stated that vertebral osteomyelitis represents 2% to 7% of all cases of osteomyelitis.17–20 The disease may occur from infancy to old age but has a predilection for the elderly.15,19,21–30 Approximately one half of the patients with spine infections are more than 50 years old and two thirds are male.29 The incidence may be higher in younger patients who are intravenous (IV) drug abusers.2
Etiology
Any condition that causes a bacteremia may lead to hematogenous vertebral osteomyelitis. The most frequent sources are urinary tract infections and the transient bacteremia caused by genitourinary procedures.16,22,24,28,31–33 Of 198 cases in the literature in which the probable source of infection was noted, it was the genitourinary tract in 29%, soft tissue infections in 13%, and respiratory tract infections in 11%; 1.5% of the infections occurred in IV drug abusers,29 but this association is being reported with increasing frequency.29,34–39 Vertebral osteomyelitis may also be caused by direct inoculation of bacteria into the spine by penetrating wounds, spine surgery, chemonucleolysis, or discography.29,40–49 The source of infection could not be identified in 37% of cases.29 Immunocompromised hosts appear to be particularly susceptible to spine infections.22,29,31,33 In particular, diabetics have a high incidence of vertebral osteomyelitis.16,21,31,50 Those who are human immunodeficiency virus (HIV) positive are also predisposed to develop spinal infections even when IV drug users are eliminated from the study group.51 In a recent series of 253 patients from the Cleveland area, 33% of infections were acquired in the hospital. In total, 51% of patients had predisposing extravertebral infections with most due to hematogenous spread from urinary tract, skin and subcutaneous tissues, infected vascular access sites, endocarditis, and bursitis of septic arthritis.52 Kulowski thought that trauma was a predisposing factor in pyogenic vertebral osteomyelitis.27 More recent studies have not supported that association.2,28,31 In Sapico and Montgomerie’s29 review of 207 literature cases in which the presence or absence of blunt trauma was discussed, in only 5% was there a history of trauma.
Bacteriology
In 1931 Hatch reviewed the literature and reported that the causative organism was almost exclusively Staphylococcus aureus.53 There has been an increase in the number of gram-negative bacillary infections.24 In the series from Cleveland, gram-negative bacilli accounted for 23% of infections.52 From data reported in the postantibiotic era, Sapico and Montgomerie29 found that 67% of 222 patients were infected with gram-positive aerobic cocci; S. aureus constituted 55% of the total. The emergence of tolerant S. aureus is a concern, and such strains may become more prevalent with the widespread use of antibiotics.54 The most frequently isolated gram-negative organisms are Escherichia coli, Pseudomonas species, and Proteus species. These are frequently found in association with genitourinary tract infection.24,31,32,55–57 Pseudomonas aeruginosa is frequently isolated from heroin abusers.34,36,38,58–60 In a review of 67 reported cases, gram-negative aerobic bacilli were isolated in 82% of the cases and Pseudomonas was the pathogen in 66%.38 However, one series included 15 IV drug abusers with pyogenic vertebral osteomyelitis and all 11 with positive cultures were infected with S. aureus.22 The likelihood of isolating an organism in the blood is most likely when fever is present. In the absence of a fever, bacteremia can only be detected in 21% of patients.52
Salmonella osteomyelitis is uncommon. It generally occurs after an acute intestinal infection, but the interval between the gastroenteritis and the onset of osteomyelitis may be quite long61; in some cases, no previous infection can be identified.62 Salmonella has a strong tendency to localize in tissues where there is preexisting disease.61,63 Infection with anaerobic bacteria is unusual and is generally associated with foreign bodies, open fractures, infected wounds, diabetes, or human bites.29,64
Infection caused by multiple organisms is encountered in up to 8% of cases.29,65 Infection with Haemophilus species has been reported but is extremely rare in adults.66,67 Low-virulence organisms such as diphtheroids and coagulase-negative staphylococci may cause indolent infections with delayed diagnosis.68 These organisms may grow slowly and cultures should be held for 10 days before they are considered to be negative. Low-virulence organisms should not be dismissed as contaminants in patients suspected clinically to have vertebral osteomyelitis.68 In one series of 111 cases of pyogenic vertebral osteomyelitis, low-virulence organisms caused 48% of the infections in the 61 patients who were 60 years of age or older and 55% of the 44 patients who had an impaired immune system.22
Pathogenesis/Pathology
Although the nucleus pulposus is an avascular tissue, it is relatively active metabolically.69 It receives its nutrition via diffusion across the endplates and from blood vessels at the periphery of the annulus fibrosus.69 In the developing spine, orderly arranged cartilage canals within the endplate contain vascular organs resembling glomeruli.70,71 Earlier studies suggested that blood vessels penetrate the nucleus pulposus in human fetuses and neonates.72 However, elegant studies by Whalen and colleagues71 demonstrated that the nucleus pulposus is always avascular. Coventry and colleagues73 demonstrated that, after birth, the cartilage endplates become progressively thinner and the vessels within the cartilage canals become obliterated. Some persist up to age 30 years, but by adulthood most of the vessels within the endplate itself have disappeared.73
Wiley and Trueta74 demonstrated the rich arterial anastomosis within the vertebral body, with end arterioles in the metaphyseal region. Spinal arteries enter the canal through the intervertebral foramen at the level of the disc. Branches ascend and descend, supplying the vertebral bodies above and below. Through their injection studies, Wiley and Trueta demonstrated how bacteria could easily spread hematogenously to the metaphyseal region of adjacent vertebrae. The infection may also start in the metaphyseal region of one vertebra and either spread across the avascular disc by lysosomal destruction of the nucleus pulposus or through vessels anastomosing on the periphery of the annulus fibrosus.74
It has been suggested that Batson’s plexus may be the route of hematogenous spread of infection. Batson demonstrated, in injection studies, that dye flows into the valveless vertebral venous plexus when pressure is applied to the lower abdominal wall.75 The distribution of veins within the vertebral body is an arborization of vessels. Minute tributaries draining the metaphyseal region empty into large, valveless, venous channels that drain into the loose plexus lining the canal. Wiley and Trueta demonstrated that it takes considerable force to fill the small metaphyseal vessels in a retrograde fashion, compared with the ease of injection of the metaphyseal arterioles; this suggests that the former is an unlikely route of hematogenous seeding.74
Once microorganisms lodge in the low-flow vascular arcades in the metaphysis, infection spreads. The disc is destroyed by bacterial enzymes in a manner similar to the destruction of cartilage in septic arthritis. This is in contrast to tuberculous infections (described later), in which the endplates and bone are destroyed but the disc is frequently better preserved.76 In children, the cartilage canals allow microorganisms nearly direct access to the disc, which probably explains the clinical differences between spine infections in children and adults. In adults, disc space infection may occur by direct inoculation of the disc as a result of surgery, chemonucleolysis, or discography but is unlikely to occur spontaneously.77,78
Some authors have suggested that discitis in adults is a separate entity from vertebral osteomyelitis.26,79 Ghormley and colleagues26,79 stressed the benign nature of this variation, but in Kemp and colleagues’26 series the disease was quite severe with a high rate of irreversible paralysis. It is conceivable that adult discs could receive blood directly through persistent vascular channels in the endplate, degenerative defects in the endplate, or vessels anastomosing on the peripheral annulus fibrosus and perhaps gaining access through rents in the annulus. If adult discitis occurs at all, it appears that hematogenous involvement of the metaphysis is far and away the most common mechanism and, whether the infection begins in the metaphysis and spreads across the disc or vice versa, the clinical manifestations and treatment are the same.
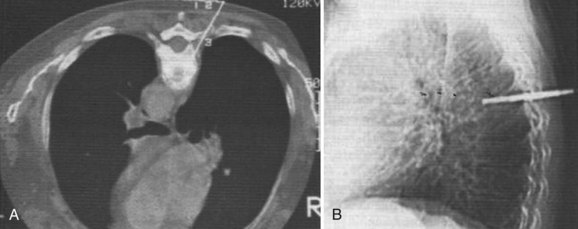
The upper cervical spine has a peculiar blood supply. Parke and colleagues80 have demonstrated a venous plexus around the odontoid, called the “pharyngeal vertebral vein,” which frequently has lymphovenous anastomoses. This venous plexus may be responsible for hematogenous spread to the upper cervical spine.80,81 Abscesses may drain into the soft tissues surrounding the spine or into the spinal canal itself. In the cervical spine, a retropharyngeal abscess may invade the mediastinum.27,82 In the thoracic spine, an abscess may be paraspinous or retromediastinal.27 Infection in the lumbar spine may cause a psoas abscess or, less commonly, an abscess pointing through Petit triangle.27 Occasionally, an abscess may create a tract through the greater sciatic foramen and appear in the buttock beneath the piriformis fascia, in the perirectal region, or even in the popliteal fossa.27 The more virulent organisms may not follow fascial planes and may extend into visceral structures. They also are more likely to produce spinal deformity. An abscess that enters the spinal canal is considered to be an epidural abscess and is discussed later. Infection may cross the dura, causing a subdural or intradural abscess or meningitis.27,83
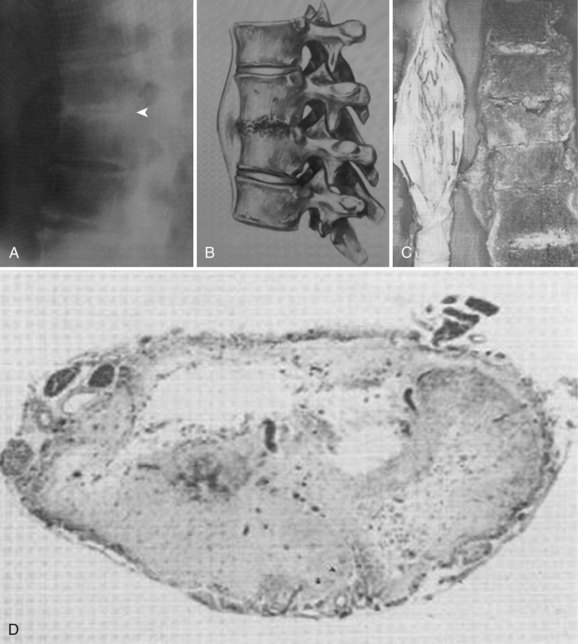
The pathogenesis of neural compromise may be related to direct compression by epidural pus, granulation tissue, or bone and disc from the development of spinal deformity and instability. In addition, the cord or nerve roots may suffer ischemic damage from septic thrombosis or may be damaged by inflammatory infiltration of the dura (Figs. 86-1 and 86-2).26,27,29
An unusual association between vertebral osteomyelitis and compression fractures secondary to osteoporosis has been described. It is theorized that the osteomyelitis may develop as a complication of the fracture because the fracture creates a favorable environment for the hematogenous infection. Alternatively, the osteomyelitis may develop within the central portion of an osteoporotic vertebral body, perhaps because the bone is more hyperemic or because of vascular stasis. Infection may then lead to a pathologic fracture of the vertebra without the usual involvement of the disc space.84
Clinical Presentation
The clinical manifestations of spine infection are determined by the virulence of the organism and the resistance of the host. The presentation may be acute, subacute, or chronic.26,27 Before the antibiotic era, most patients had acute osteomyelitis, and in 68% of the cases the disease was fulminant with severe toxemia.53 The mortality rate ranged from 25% to 71%.27,53 A literature review in 1979 found that only 20% of the patients had symptoms for less than 3 weeks before presentation, 30% had them for 3 weeks to 3 months, and 50% had them for longer than 3 months.29 Greater awareness of the disease and improved diagnostic modalities (especially magnetic resonance imaging [MRI]) have shortened the delay in diagnosis. In one series reported in 1997, 68 of 111 patients were diagnosed within 28 days of the onset of their symptoms and only 8 patients were diagnosed more than 3 months after their symptoms began.22 In an urban setting, the infection is discovered within 1 month in only 28% and the median time to diagnosis is 1.8 months.52
Fever is present in only 52% of the patients overall; pain in the back or neck is a much more common finding, occurring in approximately 90% of patients.29 Patients with acute infection present with fever, local spine pain, severe muscle spasm, and limitation of motion of the spine. With lumbar spine involvement, there may be a positive straight-leg raise test, reluctance to bear weight, and hip flexion contracture due to psoas irritation. Hamstring tightness and loss of lumbar lordosis may be noted. Torticollis and fever may be the only presenting signs with cervical osteomyelitis.81,85
Subacute and chronic infections may be much more insidious, and these patients have a vague history. Pain may be the only symptom, especially with an occult infection by a low-virulence organism.68 Approximately 15% of the patients have atypical symptoms such as chest pain, abdominal pain, hip pain, radicular symptoms, or meningeal irritation.16,29,86 These unusual and often vague complaints have led to unnecessary exploratory laparotomies before the diagnosis has been made.29,86 A significant delay in diagnosis is common with chronic infections.16,21,23–25,29,87,88
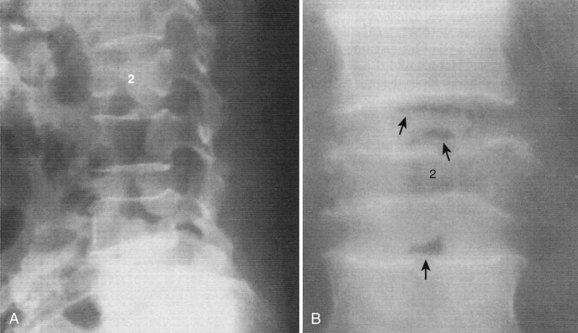
Vertebral osteomyelitis is more common in the lumbar region. In Sapico and Montgomerie’s29 literature review, in 48% of 294 cases the involvement was lumbar, in 35% thoracic, in 6.5% cervical, and in approximately 5% thoracolumbar and lumbosacral. Vertebral osteomyelitis at noncontiguous levels is uncommon (Fig. 86–3). With the advent of antibiotics, significant spine deformity is not as common as it was in the past, but significant kyphosis still may occur.24,27
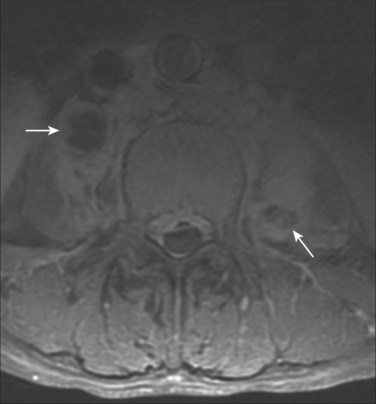
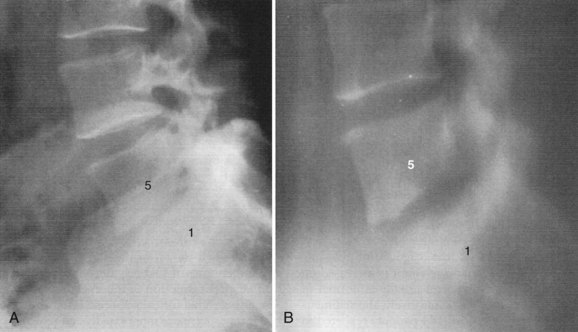
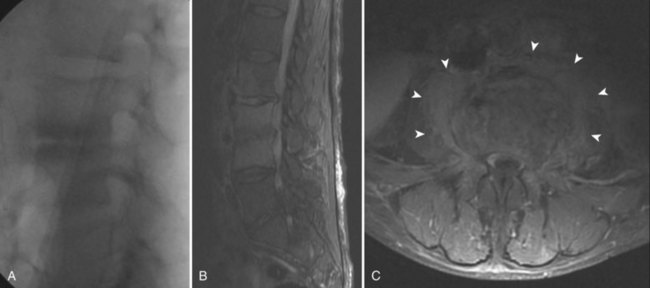
Abscesses are not encountered as frequently now as they were before the antibiotic era but should still be sought, in the paraspinous region, psoas region, and in remote areas.27 A tender or pulsatile abdominal mass may be caused by a mycotic aneurysm, a dilatation of the wall of an artery resulting from an infection.89 In the lumbar spine, abscesses in the psoas muscle are common and help to distinguish spinal infections from other lesions such as tumors or trauma (Fig. 86–4).
Approximately 17% of the patients present with a neurologic deficit secondary to nerve root or spinal cord involvement.29 Eismont and colleagues identified several factors that predisposed patients to paralysis including diabetes,16,27,50,90 rheumatoid arthritis,90 increased age,90,91 and a more cephalad level of infection.37,90 Patients on systemic steroid therapy are more likely to be paralyzed, and those infected with S. aureus seem to have the most severe degree of paralysis.90 Some authors have noted that neurologic involvement is uncommon in patients infected with Pseudomonas.58,60
Infants and IV drug abusers are two subsets of patients who have slightly different presentations. Infants generally present acutely with high temperature, septicemia, and generalized signs of systemic illness.91,92 The radiographic findings of vertebral osteomyelitis in infants is striking, with almost complete dissolution of the involved vertebral bodies and nearly normal adjacent endplates. The late radiographic appearance may be identical to that of congenital kyphosis (Fig. 86–5). Heroin abusers also present earlier than most patients. In a review of the literature, 81% of heroin abusers presented within 3 months after the onset of their symptoms, compared with 50% in the general population with vertebral osteomyelitis.29,38,91,92 The authors postulated that the earlier presentation may be related to infection with more virulent organisms or the fact that their patients have less tolerance to pain or may be using their back pain as an excuse to receive more narcotics (Fig. 86–6).38
Diagnostic Evaluation
Laboratory Evaluation
The erythrocyte sedimentation rate (ESR) and Gram stain and culture are commonly used laboratory tests in the diagnosis of pyogenic spine infections.23,29,31,77 The leukocyte count is increased on presentation in only 42% of cases and is usually normal in patients with chronic disease.29,31,77 Conversely, the sedimentation rate was increased in 92% of 184 patients reported in the literature.29 It is a nonspecific test, however, and the rate may be increased in pregnancy, malignancy, other infections, dysproteinemias, and connective tissue diseases. In addition, it is influenced by serum levels of fibrinogen and globulin.29 The sedimentation rate may be normal in occult infections with low-virulence organisms.68
C-reactive protein (CRP) has been shown to be helpful in the diagnosis of postoperative discitis 93 and has supplanted ESR as the laboratory study of choice for assessing the presence of infection. CRP was discovered in 1930 by Tillett and Francis in their studies of pneumonia. This acute phase protein is a 187 amino peptide that can precipitate the C-fraction polysaccharide from Streptococcus pneumonia.94 With the onset of an infection, or other inflammatory process, the CRP level increases within 4 to 6 hours, doubling every 8 hours, and peaking at about 36 to 50 hours.94 The half-life of CRP is 24 to 48 hours. The degree of increase can be up to 10,000-fold higher than normal, while ESR increases only 10- to 100-fold.95 The ESR begins to increase only several days after onset of infection and peaks at 7 to 8 days.94 An additional limitation of the ESR is the long period of recovery. The ESR has been useful in follow-up to assess the response to treatment.15,23,24,26,29,96,97 In one small series, the sedimentation rate decreased to at least two thirds of the original value at the completion of successful antibiotic therapy in all patients and decreased to half of the original value in the majority.29 In another series of 30 cases, the sedimentation rate returned to normal after resolution of the infection.23 However, the ESR remains elevated for more than 3 weeks postinfection.98 The CRP, in contrast, decreases more rapidly and returns to normal levels in 10 days. In cardiac patients, elevated preoperative CRP increases the risk of postoperative infectious complications.99 Similar to ESR, the CRP unfortunately suffers from low specificity because any infectious or inflammatory process will elevate these values.100 After routine spinal surgery, the CRP returns to normal in 5 to 14 days, whereas the ESR takes 21 to 42 days to return to normal.101 Thus an abnormal increase in CRP 1 to 2 weeks after spinal surgery may represent onset of postoperative infection. In all cases, a blood culture should be obtained because it remains a convenient, readily accessible means of identifying an organism. In most cases the identification of the offending organism correlates well with biopsy results. In a study of 29 cases of pyogenic spondylitis, O’Daly and colleagues102 found a 100% correlation between blood cultures and vertebral cultures when both were positive.
Imaging Studies
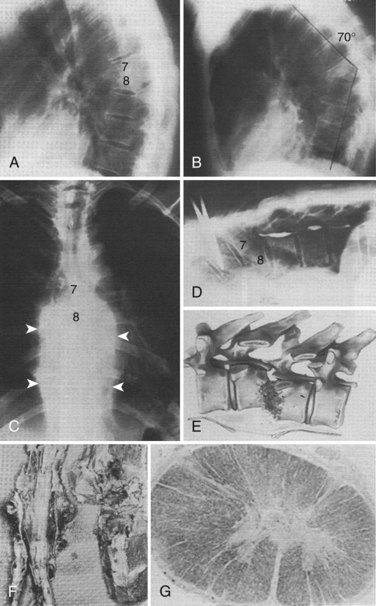
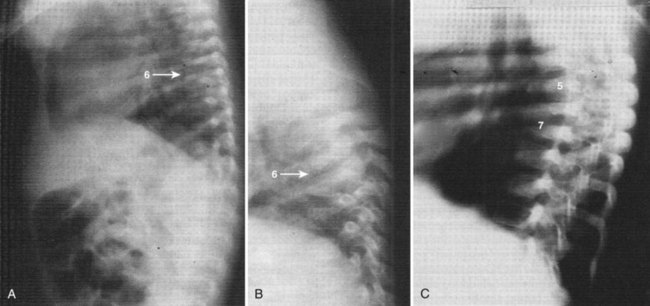
The findings on plain radiographs are characteristic but do not appear for at least 2 to 4 weeks.23,28,30,31,56,76 The earliest and most constant radiographic finding, narrowing of the disc space, is present in 74% of patients at presentation.29 Tomograms show abnormalities earlier than plain radiographs (Fig. 86–7) and may show local osteopenia of the endplates at 10 to 14 days, but tomography has largely been supplanted by computed tomography (CT).31 Widening of the retropharyngeal space in the cervical spine, enlargement of the paravertebral shadow in the thoracic spine, or changes in the psoas shadow in the lumbar spine may indicate either abscess or granulation tissue surrounding the infection. After 3 to 6 weeks, destructive changes in the body can be noted, usually beginning as a lytic area in the anterior aspect of the body adjacent to the disc and diffusely in the endplate.
Reactive bone formation and sclerosis are present in 11% of patients on presentation, and most patients will have sclerosis when the disease heals.29 Depending on the virulence of the organism and the response to treatment, progressive bony destruction, collapse, and kyphosis may develop. The radiographic findings generally lag behind the clinical response by 1 to 2 months. With healing, new bone formation and hypertrophic changes at the vertebral margins eventually may produce spontaneous fusion. Fusion occurs in just over 50% of the cases29,103 but may take up to 5 years.104 If a solid fusion does not occur, a fibrous ankylosis may be achieved.24,103
Although the radiographic findings are characteristic, they are not specific and a definite diagnosis is possible only by biopsy.103 An unusual radiographic finding that may help with the diagnosis is gas in the disc space; this may represent infection with a gas-forming organism (Fig. 86–8).65 However, the most common cause of gas in the disc space in adults is due to degenerative disease.
An atypical presentation of vertebral osteomyelitis was reported by McHenry and colleagues.84 They described a series of six patients with osteomyelitis in an osteoporotic vertebral compression fracture. The vertebral endplates were intact on the initial plain radiographs.84 This presentation occurred in 13% of all hospitalized patients with vertebral osteomyelitis and 2.4% of inpatients with osteoporotic compression fractures at one institution over a 5-year period.105 Chest radiographs may reveal atelectasis, pleural effusion, and soft tissue masses that may be confused with a tumor.87
Radionuclide studies are useful for early detection and localization of infection, before plain films become positive.24,26,30,88,97,106–108 Clinical studies have suggested that gallium scans become positive before technetium scans do,106 and this has been confirmed in experimental studies.109 Technetium scans show increased uptake diffusely in the region of the infection, whereas gallium scans may show increased uptake in a butterfly area around the infected spine.110 Gallium scanning has been found to have a sensitivity of 89%, a specificity of 85%, and an accuracy of 86% in the diagnosis of disc space infections.106 In a separate study,107 technetium scans were found to have a sensitivity of 90%, a specificity of 78%, and an accuracy of 86%. The accuracy of combined technetium and gallium scans was 94%.107 These two scans combined are currently the authors’ preferred nuclear medicine studies.
In experimental disc space infection, bone scans were positive in 23% at 3 to 5 days, in 29% at 6 to 8 days, and in 71% at 13 to 15 days.111 The probability of technetium bone scans becoming abnormal increases with the duration of symptoms, to almost 100%, but false-negative scans have been reported in young children and also in the elderly. This has been postulated to be the result of regional ischemia.68
The major mechanism of gallium localization is thought to be neutrophil labeling followed by migration to the inflammatory focus. False-negative gallium scans have been reported in leukopenic patients.108 Both technetium and gallium scans may be negative with occult infection by low-virulence organisms.68 Two cases have been reported in which the technetium scan was negative but the gallium scan was positive, and the authors postulated that this represented pyogenic discitis without vertebral osteomyelitis.112 Technetium scans remain positive for a long time after resolution of the disease, whereas gallium scans become normal during healing and, therefore, may be useful in following the response to treatment.113
Indium-111-labeled leukocyte imaging has been found to be helpful in the evaluation of sepsis in the appendicular skeleton.114 Unfortunately, it is not sensitive in the spine.115–117 This may be related to the fact that most cases of vertebral osteomyelitis are chronic by the time the patients are studied, and the inflammatory response may have fewer leukocytes. The overall sensitivity of indium scanning for infections of the spine is 17%, the specificity is 100%, and the accuracy is only 31%.116 A correlation was found between prior antibiotic therapy and false-negative indium scans and photon-deficient indium uptake.116 Photon-deficient lesions may be detected by indium-111-labeled leukocyte imaging in many other conditions including previous surgery, radiation therapy, or metastatic disease.118 Palestro and colleagues119 reported that the specificity was 52% and the sensitivity was 54% when decreased activity was the criteria for osteomyelitis with indium-111 scanning. Single-photon emission computed tomography (SPECT) is a sensitive bone scintigraphic modality for early detection of spondylitis. It is more sensitive than planar scintigraphy and has the advantage of increased contrast resolution and the capability of three-dimensional localization.120 Scintigraphy with technetium and gallium are now often performed with SPECT. Love and colleagues121 compared three-phase bone technetium SPECT, with gallium SPECT, and MRI in 11 patients with spinal infections. Gallium SPECT and MRI were comparable in accuracy and superior to technetium SPECT. Thus gallium SPECT may be useful in patients in whom MRI is contraindicated or in cases where the diagnosis is uncertain. A positive technetium SPECT, in the setting of a negative gallium scan, points toward noninfectious cases of back pain such as degeneration disease or pseudarthrosis.122
CT may show cystic changes in the bone, as well as soft tissue masses, gas in the soft tissues or within the bone and disc, and, later, lytic destruction of the body.123–125 The prevertebral soft tissue involvement seen on CT usually completely surrounds the spine anteriorly, and the destruction of the vertebra is generally an osteolytic process around the disc space (see Fig. 86–6). This is in contrast to neoplasms, which are characterized by no or only partial paravertebral soft tissue swelling and by changes that may be osteoblastic and more likely to involve the posterior elements than in infection.126
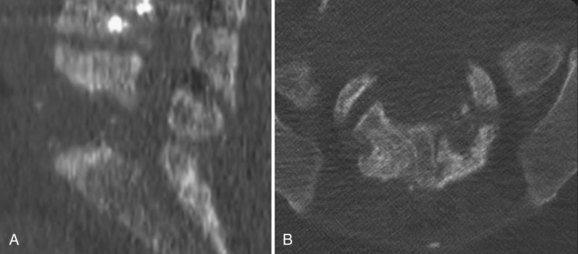
CT is valuable in differentiating pyogenic spondylitis from a tuberculous or fungal infection; in the latter, the soft tissue components tend to be more prominent.127 The finding of disc hypodensity on CT is relatively specific for infection in the lumbar spine but is less useful in the thoracic and cervical region.125 A relatively unique feature of tuberculous infection is vertebral fragmentation and paraspinal calcifications.128,129 The destruction tends to extend into the pedicle, which is uncommon in pyogenic infections. CT with contrast medium is helpful to delineate the boundary between abscesses and swollen paravertebral muscles.123–125 CT after intrathecal administration of metrizamide provides exquisite detail for the spinal canal.127 CT-guided biopsies of the spine have been shown to be safe and can be done at all levels of the spine (Fig. 86–9).123,130–132 Myelography and postmyelography CT are indicated in cases of neurologic deficit and radicular pain to rule out epidural and subdural abscesses. Cerebrospinal fluid (CSF) should also be examined to rule out meningitis.83
The imaging modality of choice for the evaluation of spine infections is MRI. MRI permits early diagnosis of infection and recognition of paravertebral or intraspinal abscesses without the risk associated with myelography.133,134 In a prospective study of 37 patients suspected clinically of having vertebral osteomyelitis, MRI was found to be at least as accurate and as sensitive as gallium and bone scanning combined: MRI had a sensitivity of 96%, a specificity of 93%, and an accuracy of 94%.107 MRI has the advantage of providing more anatomic information than radionuclide studies and is capable of differentiating degenerative and neoplastic disease from vertebral osteomyelitis.135 The changes on MRI occur at about the same time as the changes on gallium scans.107
Disadvantages of MRI are that it is more sensitive to motion degradation, and there are problems with patient positioning and claustrophobia. MRI has a limited field of view, whereas radionuclide scans can image the entire skeleton. MRI may be falsely negative in cases of epidural abscess without involvement of the adjacent bone because the signal intensity of the inflammatory exudate is similar to that of CSF.107,136
The MRI changes in vertebral osteomyelitis are characteristic (Fig. 86–10). On T1-weighted sequences, there is a confluent decreased signal intensity of the vertebral bodies and adjacent disc, making the margin between the two structures indistinct. On T2-weighted sequences, the signal intensity of the vertebral bodies and the involved disc is higher than normal, and there is generally an absence of the intranuclear cleft normally seen within the adult disc.107,133 The extent of the infection is best seen, however, using gadolinium contrast enhancement. The disc and the involved portions of the vertebral bodies reveal a marked increased signal intensity that delineates the margins of the infection (see Fig. 86–10C).137 The typical T1 changes in the vertebral body and endplates and the T2 changes in the disc space were seen in 95% of the 37 cases of vertebral osteomyelitis described by Dagirmanjian and colleagues.138 Only 56% of their cases had typical T2 vertebral body changes. Isointense or decreased signal in the vertebral body on T2-weighted images is consistent with infection if the other typical findings are present. In a more recent study by Ledermann and colleagues,139 46 patients with culture or histologic-positive spinal infections were systematically evaluated with gadolinium-enhanced MR imaging. The most sensitive MRI criteria was the presence of paraspinal or epidural inflammation (97.7% sensitivity), followed by disc enhancement (95.4% sensitivity). Hyperintensity or fluid-equivalent disc signal intensity on T2-weighted MRI was 93.2% sensitive, erosion or destruction of at least one vertebral endplate was 84.1% sensitive, and effacement of the nuclear cleft was 83.3% sensitive. When the infection is confined to a single vertebral body, spread of infection occurs in a subligamentous path.140 Interestingly, the spread of infection tends to be more commonly in a cephalad direction, affecting the superior disc space more commonly than the inferior disc space. The cause of the signal intensity changes seen in vertebral osteomyelitis is uncertain but is thought to parallel the pathogenesis of the disease. The earliest changes are thought to be related to ischemia and the increased water content of the inflammatory process. As the infection crosses the endplate, a confluent signal intensity occurs on MRI. The normal finding of an intranuclear cleft within adult discs is thought to be related to fibrous tissue within the nucleus pulposus. This cleft is lost at the time of inflammatory involvement of the disc.107
In an elegant study comparing MRI findings of pyogenic vertebral osteomyelitis with tuberculous osteomyelitis, Chang and colleagues141 identified five key distinguishing features that help to differentiate between the two disease entities. A retrospective study of 33 patients with confirmed tuberculous spondylitis were compared with 33 randomly selected patients with known pyogenic osteomyelitis. The key distinguishing features were (1) degree of bone destruction; (2) degree of disc preservation; (3) paraspinal abscess appearance; (4) abscess with postcontrast rim enhancement; and (5) postcontrast enhancement pattern of the vertebral body. As expected, the degree of vertebral body and disc destruction were the two most distinguishing differences found. Most patients in the tuberculous (TB) group (82%) had near complete destruction of at least one vertebral body, whereas less than one third (30%) in the pyogenic group had severe vertebral body destruction. Conversely, the disc was preserved in more than one half (57%) of TB group, whereas only 3% of the pyogenic group had a preserved disc space. Thus the credo that TB spondylitis “skips the disc space” is relatively well supported. However, a more accurate distinguishing feature would be better stated that pyogenic vertebral osteomyelitis differs from TB spondylitis by severe disc space destruction with relative preservation of the vertebral body.
Chang and colleagues went to further show that there were marked differences in the imaging pattern of the vertebral body itself.141 In the TB group, the enhancement pattern of the vertebral body was always focal and heterogeneous, with rim-enhancing abscesses. In contrast, the enhancement pattern of the vertebral body in the pyogenic group was nearly always (94%) diffuse and homogeneous. A discreet rim enhancement intraosseous abscess was never observed in the pyogenic group. The paraspinal soft tissue imaging patterns provide further distinguishing features. In the TB group, the paraspinal soft tissues revealed well-defined rim-enhancing lesions. In contrast, the pyogenic infections tended to show more diffuse, ill-defined areas of enhancement.
In a comparison of MRI, bone scans, and plain radiographic evaluations in an animal model of disc space infection, MRI was found to have a sensitivity of 93%, a specificity of 97%, and an accuracy of 95%, corresponding well to results of clinical studies in humans.107,111 The findings are time related. In one study, scans of rabbits made 3 to 5 days after injection of bacteria all showed a decreased signal from the nucleus pulposus on both T1-weighted and STIR (short T1-inversion recovery) sequences. Scans at 6 to 8 days also showed increased signal from the adjacent endplates on the STIR sequence and blurring of the disc margins on the T1 image. Scans at 13 to 18 days showed more florid endplate changes, and in several scans at 21 days there was increased signal from the vertebral endplates and the disc on STIR sequences.111 The MRI findings slowly return to normal after successful treatment of vertebral osteomyelitis.107 Gallium scans revert to normal much more rapidly and are better indications of appropriate therapy. Post and colleagues137 noted that abnormal gadolinium enhancement of the disc, vertebral bodies, and paraspinal soft tissues progressively decreases with successful treatment of the infection. Gillams and colleagues142,143 described some patients who were improving clinically and had stable or increasing enhancement patterns and concluded that such findings should not be interpreted as treatment failure.
Unfortunately, even MRI may be negative in surgically documented occult infections by low-virulence organisms.68 Despite the accuracy of MRI, an absolute diagnosis must be based on bacteriologic or microscopic examination of the tissue.27,144,145 The only situation in which the diagnosis can be made without a tissue biopsy is when a positive blood culture is obtained from a patient with signs and symptoms of spondylitis. Blood cultures are positive in 24% to 59% of patients with pyogenic spine infections.22,29 Urine cultures are less reliable because patients with vertebral osteomyelitis may have a coexistent urinary tract infection with a different organism.22,24
Biopsy
Needle biopsy of the spine was first reported by Ball in 1934. In 1956 Craig described a set of instruments designed to increase the percentage of successful closed-needle biopsies, especially in sclerotic or softened bone, discs, or fibrous tissue.146 Needle biopsies have been shown to be safe in the cervical and thoracic spine, as well as in the lumbar spine.147,148 A definite diagnosis is possible by closed-needle biopsy in 68% to 86% of cases.29,31,132,148,149 CT-guided closed biopsy of the spine should provide a margin of safety and allow biopsy of the area most likely to yield the diagnosis. In a series of 22 patients with a mass or destructive lesion who underwent this procedure, 17 biopsies provided a definite diagnosis; only one was false negative and in four cases the specimens were insufficient. All areas of the spine were sampled including one lesion at C2. The patient with the C2 lesion had a transient increase in quadriparesis but returned to baseline, and no other complications were reported.130
Closed biopsies of the spine are often false negative in patients who are being treated with antibiotics at the time of the biopsy. If a biopsy is nondiagnostic, it would be reasonable to observe the patient off the antibiotic regimen and repeat the biopsy if the clinical situation allows such a delay. If the second closed biopsy is also nondiagnostic, an open biopsy should be considered. This will provide larger tissue samples and selection of grossly pathologic tissue and should have a lower false-negative rate. In their review of the literature, Sapico and Montgomerie29 found that 30% of needle biopsy specimens and aspirates were sterile, compared with only 14% of surgical specimens. The technique of transpedicular biopsy allows for larger bony samples to be obtained. The transpedicular approach is safely performed with either fluoroscopy or CT. For general biopsies including tumor, the accuracy rate is over 92%.150–152 In the setting of infection, this technique yields better results than traditional needle biopsy. Hadjipavlou and colleagues153 examined 28 patients with suspected spinal infections diagnosed by a combination of laboratory test, MRI, and scintigraphy. Positive cultures were obtained in 71% of the biopsies.153
The differential diagnosis of pyogenic vertebral osteomyelitis includes tuberculosis, fungal infections, metastatic carcinoma, multiple myeloma, localized Scheuermann disease, trauma, degenerative disease, epidural abscess, and fractures associated with osteoporosis.15,29,84,154 Less common disorders in the differential diagnosis are leukemia, perinephric abscess, neuropathic spinal arthropathy, and sarcoidosis, as well as erosive arthritides in rare cases of facet joint involvement.28,77,92,155–157 With such a wide variety of diseases that can present with signs and symptoms similar to those of vertebral osteomyelitis, diagnostic acuity is important. As Kulowski said in 1936, “Knowledge of the disease is the primary factor in the diagnosis.”27
Management
Before the advent of antibiotic therapy, treatment of pyogenic vertebral osteomyelitis involved drainage of abscesses, rest on a frame or plaster bed, and attention to nutrition and general hygiene. The mortality rate with this approach was between 25% and 70%.27,53 The use of antibiotics has drastically changed the prognosis with this disease, but attention to good general medical care is still a vital part of the treatment. Associated conditions that compromise wound healing or immune response should be managed aggressively. Attention to proper nutrition and the reversal of metabolic deficits and hypoxia are essential. Diabetes and other systemic illnesses should be brought under control.90 Any underlying focus of infection in the urinary tract, lungs, skin, or elsewhere must be treated concurrently with the spine infection.158
Daly and colleagues159,160 have demonstrated that antibiotic penetration of osteomyelitic bone parallels serum concentrations for all classes of antibiotics. The penetration of antibiotics into inflammatory exudates and the intervertebral disc is less certain.161–166 Vancomycin, gentamycin, tobramycin, clindamycin, and teicoplanin all penetrate the nucleus pulposus reasonably well.161,167 The data regarding cephalosporins are inconclusive, but if penetration does occur, it appears to be at a relatively low level.162,165,166 The penetration of cephalosporins into inflammatory exudates appears to be inversely proportional to the degree of serum protein binding.163 Riley and colleagues168 have shown that the penetration and distribution of antibiotics into the nucleus pulposus is significantly influenced by the charge of the antibiotic with positively charged molecules penetrating the best.
The route of administration of the antibiotics and the duration of therapy are somewhat empiric because little research has been done to clarify these topics. At present, it is recommended that parenteral antibiotic therapy be used in maximal dosage for 6 weeks and followed with an oral course of antibiotics until resolution of the disease. It may be reasonable to switch from parenteral to oral therapy at 4 weeks.24 Parenteral therapy for less than 4 weeks results in a higher rate of failure.29 Oral ciprofloxacin therapy has been used successfully in the management of patients with chronic osteomyelitis of the tibia or femur.169 It is possible that ciprofloxacin and other new agents for oral use may supplant parenteral treatment of vertebral osteomyelitis in the future, but general use of these agents should await evidence of their effectiveness.
The erythrocyte sedimentation rate has been found to be a reasonable guide to the response to therapy15,23,24,26,29,96,97 and can be expected to decrease to half to two thirds of pretherapy levels by the completion of successful treatment.29 If the sedimentation rate does not decrease with treatment, consideration should be given to a repeat biopsy. CRP is a more useful laboratory test to follow resolution of the infection, as described earlier. However, it is still unclear at what point to discontinue antibiotics on the basis of CRP levels. Further studies are necessary to correlate CRP levels with the duration of antibiotic treatment. Currently, at least 6 weeks of parenteral antibiotics is used empirically. Antibiotic administration must be carefully monitored to avoid toxicity, especially in diabetics and other who might have impaired renal function.50
Patients should be immobilized for pain control and prevention of deformity or neurologic deterioration. The length of time a patient should remain at bed rest, the type of orthosis, and the duration of its use all depend on the location of the infection in the spine, the degree of bone destruction and deformity, and the response to treatment. Thoracic and thoracolumbar lesions may require bed rest on a RotoRest or similar device if there is undue pain. Rigid bracing with a thoracolumbosacral orthosis (TLSO) suffices in most cases to allow mobilization of the patient. Thoracic and thoracolumbar lesions are more likely to cause deformity and, if neurologic deficits occur, the prognosis is worse with these lesions than with lumbar spine involvement.24,90
Cervical and cervicothoracic lesions may be immobilized with a halo device if there is significant instability or deformity. In most cases external bracing is sufficient, and often better tolerated by the patient. Upper thoracic lesions are best immobilized in a TLSO device with a chin piece, and lower thoracic and lumbar lesions should be immobilized in a TLSO device without a chin piece. Frederickson and colleagues24 found that immobilization was most important in those patients with destruction of greater than 50% of a vertebra and recommended immobilization for the first 3 months. In 5 of their 17 cases, significant deformity developed in the first 6 to 8 weeks, all at the thoracolumbar junction or above; those patients with the greatest deformity had 50% or more vertebral body destruction at presentation.106 Most authors recommend bracing for at least 3 to 4 months. Garcia and Grantham31 recommended that the duration of immobilization should be individualized and based on the response to treatment.
Surgical Treatment
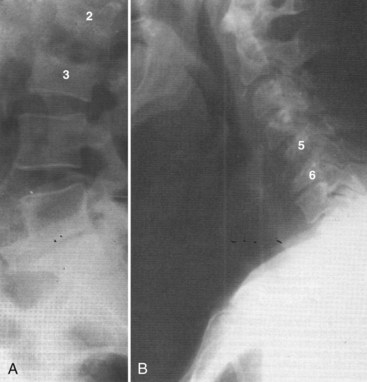
Surgery is indicated in the following circumstances: (1) to obtain a bacteriologic diagnosis when closed biopsy is negative or deemed unsafe; (2) when a clinically significant abscess is present (spiking temperatures and septic course); (3) in cases refractory to prolonged nonoperative treatment, where the ESR and/or CRP remain high or pain persists; (4) in cases with spinal cord compression causing a neurologic deficit; and (5) in cases with significant deformity or with significant vertebral body destruction, especially in the cervical spine.90,170,171 Upper cervical osteomyelitis is rare but generally requires fusion because of the associated instability.81
In cases of lumbar lesions with root deficits, the final outcome is satisfactory with or without surgical treatment, but patients with spinal cord compression have a better prognosis with surgery.90 Surgery should be carried out as soon as possible in these cases, but when doubt exists regarding the chances of a reversible spinal cord lesion, decompression should be carried out because recovery has been noted in patients with paralysis who underwent decompression as late as 5 months after the onset of weakness.90
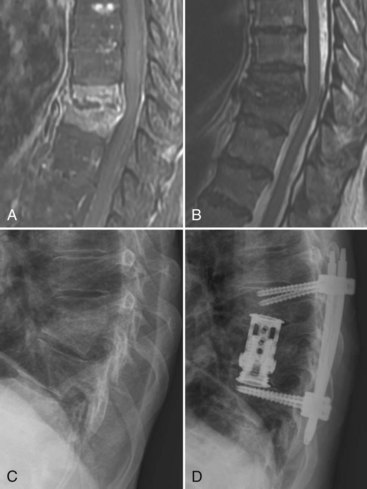
In most cases the spine should be approached anteriorly because this allows direct access to the infected tissues and adequate débridement. Anterior exposure allows stabilization of the spine by bone grafting, which promotes rapid healing without collapse and assists rehabilitation (Fig. 86–11).90,172–176 Laminectomy without anterior débridement and reconstruction is contraindicated in most cases because it may lead to neurologic deterioration and increased instability.90,173,177 The situation is similar to that in acute trauma.178 Laminectomy may be performed in the lumbar spine below the level of the conus, provided there is no psoas abscess or extensive anterior destruction of the bodies that would require débridement.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree