16
Ulnar Tunnel Syndrome
Robert M. Szabo
History and Clinical Presentation
A 59-year-old right hand dominant physician fell off a curb on the outstretched hand 14 months prior to this evaluation. At that time, he was treated for a fracture at the base of his right fifth metacarpal and a fracture of his left radial neck. He noticed paresthesias along the ulnar side of his right fifth finger and right-sided hand weakness. The patient is a non–insulin-dependent diabetic who suffered a subarachnoid hemorrhage complicated by uncontrolled seizures, for which he had a hippocampectomy and still takes carbamazepine (Tegretol).
PEARLS
- Routine release of the ulnar tunnel in patients with carpal tunnel syndrome and mild ulnar symptoms is not indicated; carpal tunnel release alone increases the volume of the ulnar tunnel and changes its shape from triangular to ovoid.
- Anatomy in this area is quite variable, so always start dissection proximal to the wrist crease and proceed distally and anticipate anomalies.
- Clawing of the small and ring fingers is seen much earlier with compression of the ulnar nerve at the wrist than at the elbow because of the intact flexor digitorum profundus innervation.
PITFALLS
- Patients with coexisting polyneuropathy may benefit from ulnar tunnel release but should be warned that not all of their symptoms may resolve.
- Do not cross the wrist crease with a perpendicular incision or a hypertrophic scar may form.
Physical Examination
Point tenderness was noted over the hook of the hamate. There were no other areas of tenderness, signs of previous trauma, or any swelling, masses, or bruits. Sensibility testing with Semmes-Weinstein monofilaments revealed 3.61 in the little finger and ulnar half of the ring finger and 2.44 in the remainder of the hand. Two-point discrimination was 7 mm in the little finger and ulnar half of the ring finger and 6 mm in the remaining fingers. Light-touch perception was normal on the dorsal-ulnar aspect of the hand and wrist. Nerve percussion and Phalen’s tests were negative. Manual motor testing was 5/5 throughout except for 0/5 of the third palmar interosseous muscle and the first dorsal interosseous muscle, which was 2/5 with moderate atrophy present. Mild clawing of the ring and small fingers was present, as was a positive Froment-Jeanne sign. Wrist range of motion was symmetric except for flexion, which was 45 degrees on the right compared with 80 degrees on the left. Key pinch on the right was 0 lb compared with 7 lb on the left. Tip pinch was 2 lb on the right and 7 lb on the left. Grip strength at the second setting was 60 lb on the right and 65 lb on the left. The Adson’s, hyperabduction, military brace positioning, and 3-minute elevation, as well as the Allen’ test, were normal.
Diagnostic Studies
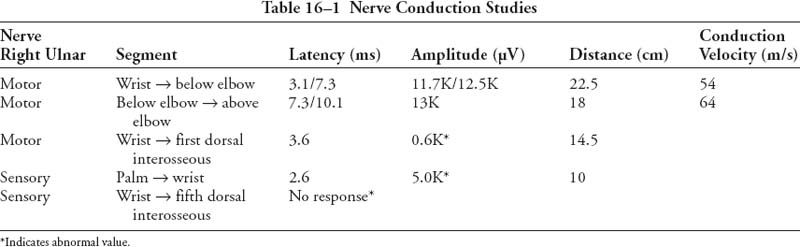
Anteroposterior, lateral, and oblique radiographs and a carpal tunnel view of the right hand revealed no acute fractures, subluxations, or dislocations. A chest radiograph was normal. Computed tomography of the wrist revealed small bone fragments adjacent to the base of the fifth metacarpal predominantly along its palmar-radial aspect. The hook of the hamate appeared intact. In the presence of a normal Allen’s test, Doppler studies were not performed. Nerve conduction study results are shown in Table 16–1.
Electromyographic Study Results
There was no evidence of abnormal spontaneous activity in the right flexor digitorum profundus, abductor digiti minimi, or the abductor pollicis brevis. In the right first dorsal interosseous muscle there was increased insertional activity with 2+ (present at multiple sites) fibrillations and 2+ positive sharp waves; recruitment frequency was 30 to 40 Hz (normal: 5 to 15 Hz); the interference pattern was decreased and discrete, indicating marked loss of motor units; amplitude was decreased with the first recruitment = 1000 μV and the duration of the voluntary motor unit potentials was increased.
Differential Diagnosis
Brachial plexus injury
Upper plexus
Lower plexus
Cervical root compression
Ulna nerve tunnel compression
Elbow
Wrist
Corvical disk disease
The ulnar nerve, containing fibers from the ventral rami of C8 and T1, is the terminal branch of the medial cord of the brachial plexus. Pathologic compression of the ulnar nerve occurs most commonly either at the elbow (cubital tunnel syndrome) or at the wrist where the ulnar nerve passes through the confines of the canal of Guyon (ulnar tunnel syndrome). With either of these conditions the patient may present with numbness along the little finger and ulnar half of the ring finger, often accompanied by weakness of grip, particularly in activities in which torque is applied to a tool, and rarely by wasting of the intrinsic musculature in the hand. The site of the compression may be determined by a careful physical examination; pain at the medial aspect of the elbow, a positive percussion test at the cubital tunnel, or exacerbation of symptoms by full flexion of the elbow suggests cubital tunnel syndrome. Sensory involvement on the ulnar dorsal aspect of the hand also suggests cubital tunnel syndrome, as the dorsal cutaneous branch of the ulnar nerve originates proximal to the canal of Guyon. Weakness of the deep flexors to the ring and little fingers as well as weakness of the flexor carpi ulnaris signal proximal ulnar nerve entrapment. Grip and pinch strength measurements may demonstrate weakness in more advanced lesions.
Sensory examination of the hand including the dorsum should be performed using Semmes-Weinstein monofilaments. Sensibility testing is an important part of the workup of a patient with an ulnar nerve compression lesion. Much of the misunderstanding over which test is better at detecting an abnormality has been cleared up with our understanding the fundamental nature of what each test is measuring. Four sensory tests are available which test different fiber populations and receptor systems. Touch fibers, group Aβ, can be divided into slowly and quickly adapting fiber systems. A quickly adapting fiber signals an on-off event, and a slowly adapting fiber continues its pulse response throughout the duration of the stimulus. Static two-point discrimination and Semmes-Weinstein monofilament tests evaluate the slowly adapting fibers, whereas vibration and moving two-point discrimination tests evaluate the quickly adapting fibers. Each fiber system is associated with a specific sensory receptor. Each clinical test of sensibility is related to a receptor group and is classified as either a threshold test or a test of innervation density. A threshold test measures a single nerve fiber innervating a receptor or group of receptors. An innervation density test measures multiple overlapping peripheral receptive fields and the density of innervation in the region being tested. Static and moving two-point discrimination are innervation density tests, which require overlapping of different sensory units and complex cortical integration. These are reliable tests in assessing functional nerve regeneration after nerve repair where brain input is radically altered but are not sensitive to the gradual decrease in nerve function seen in nerve compression. Cortical organization is intact in compression neuropathy, as the integrity of the sensory relay system remains uninterrupted. Two-point discrimination may remain intact even if only a few fibers are conducting normally to their correct cortical end points. Semmes-Weinstein monofilament and vibration tests are threshold tests and are more likely to detect a gradual, progressive change in nerve function as a greater proportion of large nerve fibers are lost whereas smaller fibers maintain their normal central connections. Clinically, threshold tests are clearly more sensitive in evaluating compressive neuropathies. At present, Semmes-Weinstein monofilament testing is simpler, less expensive, and equally as reliable and sensitive as vibration testing.
The value of nerve conduction studies is that often they provide the only objective evidence of the neuropathic condition. Electrodiagnostic testing remains the diagnostic gold standard, yet it entails several pitfalls. It is highly operator dependent, and so should be done with the same equipment and operator each time. Nerve conduction velocities and latencies can be compared with published population norms, to the contralateral nerve, to other nerves in the same extremity, or to previous tests in the same patient. Systemic conditions (including age-dependent alterations in nerve conduction) may confound the comparisons. Decreased dorsal ulnar sensation can help localize a lesion to be proximal to the wrist. Motor examination should carefully grade the flexor carpi ulnaris, flexor digitorum profundus, little and ring fingers, and the intrinsic muscles of the hand. One must keep in mind that median nerve fibers may supply some of the intrinsic hand muscles in 7.5% of limbs via a Martin-Gruber anastomosis.
Plain radiographs in two orthogonal planes should be obtained to rule out post-traumatic deformity, neoplasm, cervical ribs, or other possible bony causes of the nerve condition. A carpal tunnel view can sometimes show a hook of the hamate fracture, but often computed tomography is needed to visualize this injury. Magnetic resonance imaging (MRI) has a specific role in the workup of this condition. For instance, MRI can be extremely helpful in assessing the extent of a soft tissue mass like a ganglion causing ulnar tunnel syndrome. An apical tumor of the lung can also compress or invade the inferior brachial plexus, causing ulnar nerve symptoms. A chest x-ray to rule out a Pancoast tumor should be obtained whenever the patient gives a history of smoking, ulnar nerve symptoms, and shoulder pain.
Diagnosis
In 1861 Guyon, a French urologist, described a “loge” or a space in the hypothenar region of the wrist where the ulnar nerve bifurcates, and he prophesied compression of the ulnar nerve could be found here. Written in French, his word loge was subsequently translated to mean “canal,” and thus originated the name for the anatomic region we now call Guyon’s canal. Entrapment at this level may present with pure motor, sensory, or mixed symptoms depending on the precise location of compression. Space-occupying bony or soft tissue lesions may be causative; ganglia and other soft tissue masses are responsible for 32% to 48% of cases of ulnar tunnel syndrome. Another 16% of cases are due to muscle anomalies. Ganglia arising from the triquetrohamate joint are responsible for over 85% of the nontraumatic causes of ulnar tunnel syndrome. Other causes of ulnar tunnel syndrome include thrombosis or pseudoaneurysms of the ulnar artery, edema secondary to burns, and inflammatory arthritis.
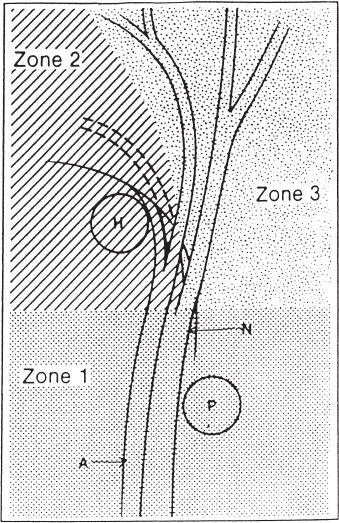
The distal ulnar tunnel, which is 4 to 4.5 cm in length beginning at the proximal edge of the palmar carpal ligament and ending at the fibrous arch of the hypothenar muscles, is divided into three zones to allow more accurate localization of ulnar nerve compressive lesions (Fig. 16–1). Zone 1 is the area proximal to the bifurcation of the nerve. Beginning at the edge of the palmar carpal ligament, it is ∼3 cm in length, bounded dorsally by the flexor digitorum profundus and transverse carpal ligament, palmarly and laterally by the palmar carpal ligament, and medially by the pisiform and flexor carpi ulnaris. Compression in zone 1 causes combined motor and sensory deficits and is most likely due to ganglions or fractures of the hook of the hamate but has been reported to occur from an anomalous muscle. Even though the hook of the hamate is in zone 2, compression of the ulnar nerve by fracture just proximal to its bifurcation can produce mixed motor and sensory symptoms. Compression in this zone has also been reported to occur from an anomalous arching pattern of the ulnar nerve piercing the flexor carpi ulnaris tendon. Zones 2 and 3 travel alongside each other from the bifurcation of the ulnar nerve to just beyond the fibrous arch of the hypothenar muscles. Although bifurcation is the most common pattern, the ulnar nerve may trifurcate at this point into two common digital sensory branches and a motor branch. Zone 2 is bounded palmarly by the palmaris brevis muscle, fibrous arch, and hypothenar muscles; dorsally by the pisohamate and pisometacarpal ligaments, triquetrohamate joint, and opponens digiti minimi muscle; laterally by the transverse carpal ligament, flexor digiti minimi muscle, and the hook of the hamate; and medially by the superficial branch of the ulnar nerve and the abductor digiti minimi muscle. Zone 2 surrounds the deep motor branch, and compression in this region produces motor deficits without sensory disturbances. Again, ganglions and fractures of the hook of the hamate are the most likely causes, but an anomalous intrinsic muscle can also be responsible for symptoms.