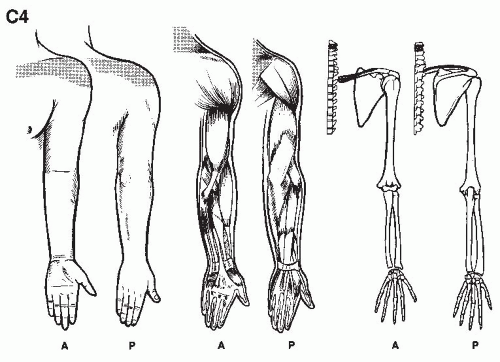
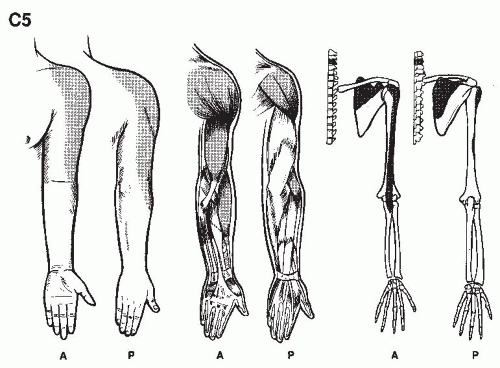
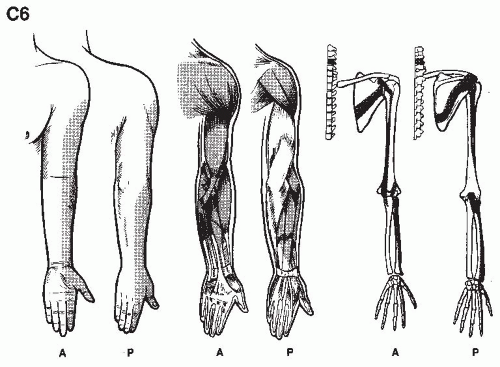
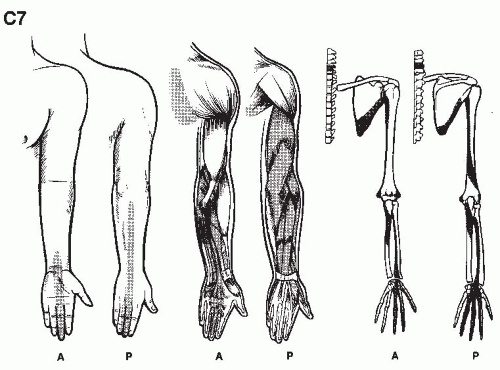
We are but dwarfs standing on the shoulders of Giants (1,2).
Pain is purely subjective, difficult to define, and often hard to characterize or interpret. It is currently defined as an unpleasant sensory and emotional response to a stimulus associated with actual or potential tissue damage or described in terms of such damage (
3,
4,
5). However, pain has never been shown to be a simple function of the amount of physical injury; it is extensively influenced by anxiety, depression, expectation, and other psychological and physiological variables. It is a multifaceted experience, an interweaving of the physical characteristics of the stimulus with the individual’s motivational, affective, and cognitive functions. The pain experience is in part behavior based on an interpretation of the event, influenced by present and past experiences.
Acute pain is a biologic symptom of an apparent nociceptive stimulus, such as tissue damage that is due to disease or trauma that persists only as long as the tissue pathology itself persists. The pain may be highly localized or may radiate. Acute somatic pain may be well localized, aching, and sharp, whereas acute visceral pain may be burning, cramping, and radiating. It is usually associated with upregulated sympathetic activity, tachycardia, hypertension, tachypnea, increased metabolic rate, and hypercoagulability. Acute pain is generally self-limiting, and as the nociceptive stimulus lessens, the pain decreases. Acute pain usually lasts a few days to a few weeks (
4). If it is not effectively treated, it may progress to a chronic form.
Chronic pain is a disease process in which the pain is a persistent symptom of an autonomous disorder with neurologic, psychological, and physiologic components. Differing significantly from acute pain, it is defined as pain lasting longer than anticipated (greater than 3 months) within the context of the usual course of an acute disease or injury. The pain may be associated with continued pathology or may persist after recovery from a disease or injury. Due to the complex nature of chronic pain, many other definitions have been proposed. Some include factors such as the persistence of pain despite extraordinary measures in a nonacute setting or pain that is without apparent biological value (
6). Operational definitions include aspects such as pain sensation, pain behavior, functional status, emotion, and somatic preoccupation (
6). As with acute pain, treatable chronic pain that is due to organic disease is managed by effectively treating the underlying disorder; however, no such identifiable organic disease may be evident. Chronic pain can mimic the qualities of acute pain except that associated signs of autonomic nervous system response may be absent, and the patient may appear exhausted, listless, depressed, and withdrawn. Chronic pain can have exacerbations that are triggered by progression of organic pathology, physiologic stress, or worsening emotional, social, and psychiatric problems. As these problems subside, the pain may improve. Chronic pain may also be highly persistent and reported as severe for years without remission.
Proper management of pain requires an understanding of its complexity and knowledge of the nonneurologic factors that determine its individual expression. The treatment of pain with physical modalities is as ancient as the history of humanity, but the use of interdisciplinary rehabilitation techniques has gained acceptance only within the past few decades.
ETIOLOGY
Chronic pain is not merely a physical sensation. In the affective component of chronic pain, most patients show a degree of depression, and many show anger, jealousy, and anxiety. For many individuals, depression is the primary factor in the perception or experience of pain. Fifty to seventy percent of patients with chronic pain have either a primary depression or a depression secondary to their pain syndrome. Chronic pain, with accompanying depression, often leads to extensive periods of reduced productivity as well as inactivity. Prolonged inactivity alters cardiovascular function, impairs musculoskeletal flexibility, and causes abnormal joint function (
15,
16,
17,
18). Prevention involves the encouragement of patient activity as soon as it is reasonable.
The motivational component of chronic pain is concerned with the vocational, economic, and interpersonal reinforcement contingencies that contribute to the learning of pain behavior and the maintenance of chronic pain. More than 75% of patients with chronic pain display adverse behavioral characteristics, including problems with job or housework, leisure activities, sexual function, and vocational endeavors (
19). Sleep disturbances and depression are highly associated with chronic pain. The patient also may have significant functional limitations as a result of multiple previous surgeries with little success and prolonged convalescence, disuse/physical deconditioning syndrome, or opioid medication (
20).
Chronic pain’s cognitive component involves how patients think and the part that pain plays in their belief system and view of self. The more the patient perceives pain as a signal, requiring a reduction of activity and protection of the affected part, the more difficult it is for the physician to achieve compliance with exercise, stretching, and other elements of the treatment program. The memories of pain from acute pain episodes may significantly hinder a patient’s recovery and contribute to chronic pain syndrome (
21,
22,
23). Pain is often the result of sensory input, affective state, cognition, motivational, and memory factors, which requires a multidimensional evaluation process, including treatment interventions directed at those components most responsible for the pain experience (
24,
25,
26).
Pain Pathways
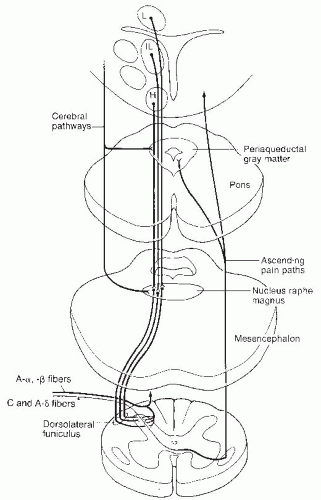
Pain is a central perception of multiple primary sensory modalities. This interpretive function is complex, involving psychological, neuroanatomic, neurochemical, and neurophysiologic factors of both the pain stimulus and the memory of past pain experiences. The peripheral mechanisms for sensing and modulating pain have been extensively studied during the past 30 years. The pathways for pain sensation, from the initial stimulus of the nociceptors to the central nervous system, are summarized in
Figure 49-1 (
27,
28,
29,
30,
31). There appear to be several descending systems that play a role in control of the modification of the ascending pain pathways, which are summarized in
Figure 49-2 (
28,
31,
32,
33,
34,
35).
Polymodal nociceptors respond to stimuli that damage tissue. This stimulation results in impulses ascending in the A-delta or C fibers to the marginal layers of the dorsal horn of the spinal cord. The A-delta fibers primarily synapse in laminae I and V, whereas C fibers synapse primarily in lamina II. Deeper regions of the dorsal horn may be polysynaptically involved in the processing of noxious stimuli.
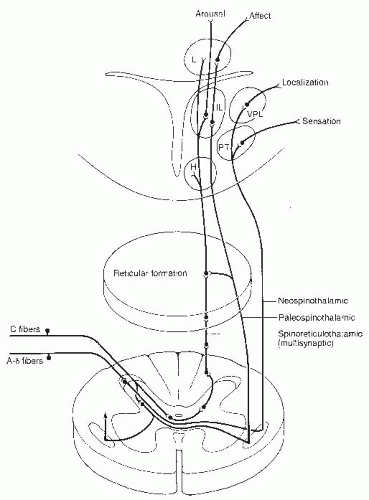
The major ascending nociceptive pathways are the spinothalamic and spinoreticular tracts, which involve both oligosynaptic and polysynaptic neurons. These oligosynaptic pathways are fast conducting, with discrete somatotopic organization resulting in rapid transmission of nociceptive information regarding site, intensity, and duration of stimulus. Further, the oligosynaptic tracts provide somatic information by way of the posterior ventral nuclei of the thalamus to the postcentral cortex. The sensory discriminatory characteristics are delineated from the neospinothalamic portion of the lateral spinothalamic tract and the nonproprioceptive portion of the dorsal columns.
Polysynaptic pathways are slow conducting, with a lack of somatotopic organization resulting in poor localization as well as dull aching and burning sensations. The nociceptive impulses transmitted through this system result in suprasegmental reflex responses related to ventilation, circulation, and endocrine function. Pathways contributing to this slow-conducting system are the paleospinothalamic tract, spinoreticular, spinocollicular, and the dorsal intercornual, as well as the spinomesencephalic tracts. The polysynaptic tracts form the brain stem reticular activating system with projections to the medial and interlaminar nuclei of the thalamus. From these nuclei, diffuse radiation occurs to the cerebral cortex, limbic system, and basal ganglia.
There are multiple levels of processing and convergence of nociceptive information in its ascending transmission to the cerebral cortex. In addition, there appear to be several descending pain control systems that play a role in the control and modification of the ascending pain pathways. The most complete studies have been of the periaqueductal gray (PAG) region of the midbrain. Stimulation of the PAG neurons and the subsequent descending impulses result in release of endogenous opioids at the nucleus raphe magnus (NRM) and nucleus locus ceruleus (NLC). Endogenous opioids activate the serotonergic cells in the NRM and norepinergic neurons in the NLC. The axons of both of these monoaminergic neurons descend in the dorsolateral tract to interneurons, predominantly in laminae I, II, and V. These monoamines activate opioid-secreting interneurons. The morphine-like transmitter released may vary, depending on what type of receptor in the periphery has been activated. Both A-delta and C afferent fibers are inhibited by descending influences in the dorsal horn. The opioid inhibitory interneurons may be influenced by intersegmental and descending pathways, but the intersegmental and segmental mechanisms have not been established. These interneurons may function either by presynaptic inhibition on the terminals with the primary nociceptive afferents preventing the release of substance P or by postsynaptic inhibition on second-order neurons. Cells in the raphe magnus are activated by ascending sensory pathways transmitted to the reticular formation as well as by descending input from cells in the PAG region.
Other descending monoamine systems include locus ceruleus to the dorsal horn interneurons, nucleus reticularis, magnicellularis to the dorsal horn interneurons, and the mesencephalic lateral reticular formation to the dorsal horn interneurons. It has been suggested that monoamines are involved with supraspinal and spinal nociceptive mechanisms. Hormonally based descending pathways have been described but are poorly understood.
Gate Control Pain Theory
The gate control theory of pain was developed by Melzack and Wall to account for mechanisms by which other cutaneous stimuli and emotional states alter the level of pain (
36). They suggested that within the substantia gelatinosa of the dorsal horn, there are interneurons that presynaptically inhibit transmission of nociceptive information to the ascending tracts. These interneurons are activated by large-diameter afferents and inhibited by small-diameter afferents. In addition, they suggested that the brain exerts descending control on this system, relying on the fact that cognitive factors are known to influence pain behavior.
Several studies have failed to provide support for the gate control theory. It remains significant, although incorrect in detail, in hypothesizing that nociceptive pain undergoes dynamic integration and modulation. The gate control theory of pain has altered the concept of pain as solely an afferent sensory experience, broadening the concept to include the affective and motivational factors involved in the human pain experience (
37). The gate control theory has been modified extensively during the past 40 years (
38). It still represents the first attempt to describe a pain-modulating system that responds to input by noxious stimuli, innocuous afferent impulses, and descending control.
Biochemical Pain Theory
In the peripheral nervous system, the extent and duration of the response to the stimulus may be modified at the biochemical level within the neurons in the pain-conducting pathway;
this neural plasticity is manifest by short-term modulation and long-term modification of the excitability of primary sensory and central neurons. Chronic pain can alter the nociceptive pathways by activation-dependent plasticity (resulting in autosensitization and windup), modulation-dependent plasticity (resulting in heterosensitization and central sensitization), or modification of primary sensory neurons, which results in pain hypersensitivity. NMDA receptors may be involved in synaptic plasticity and postsynaptic sensitization in the substantial gelatinosa and higher brain centers. This modulation of the neurotransmitters that affect the flow of information can induce the stimulation of polysynaptic neuron, or it can pathologically alter the anatomic features of these neurons and their interconnections such that the normal nociceptive stimulus response characteristics are amplified (
39).
The biochemical theory of pain has evolved since the discovery of endorphins. The endogenous opioid system consists of three families of opioid peptides: beta-endorphin, enkephalin, and dynorphin/neoendorphin. The beta-endorphins are primarily concentrated in the pituitary and the basal hypothalamus. The other endogenous opioids are distributed extensively in the central nervous system. The dynorphins/neoendorphins and enkephalins are found in the caudate nucleus, amygdala, PAG matter, locus ceruleus, and dorsal horn of the spinal cord. In addition, the enkephalins are found in the NRM and the thalamic periventricular nuclei. The dynorphins/neoendorphins are found in the hypothalamus and substantial nigra (
32).
Endogenous opioids are involved in analgesia, as well as multiple other clinical events. There are at least seven different opiate receptors, of which the µ, δ, and κ appear to be involved in analgesia (
40). The others are associated with such functions as respiration, appetite, hallucinations, dysphoria, immune function, temperature regulation, memory, and blood pressure control. Beta-endorphins may function in the modulation of local blood flow and immune function (
41). The discovery of multiple opiate receptors and multiple endogenous opioid compounds provides an explanation for the multiple effects of the endogenous opioids. Within the peripheral and central nervous systems, the enkephalins act as neurotransmitters and the β endorphins act predominately as hormones. Endogenous opioids are only one part of a complex modulatory system involved in the collating, processing, and filtering of information concerning tissue damage (
42).
Neural plasticity has been observed in patients with chronic pain. Persistent pain has been shown to increase the strength of the polysynaptic response as well as the anatomic configuration of the pain conduction pathway. This may result in long-term alteration in the connectivity and organization of pain pathways. The consequence of this alteration is a “pain memory” that is evoked with minimal stimulation and results in a maximal nociceptive response (
39,
43). Of note, neuroplasticity allows for secondary development of hyperalgesia with chronic opioid usage (
44). The modulation of pain may occur via pronociceptive or antinociceptive neurotransmitters at multiple neurons along the pain conduction pathway. Pronociceptive neurons include WW glutamate, aspartate, and substance P. Antinociceptive transmitters include gamma-aminobutyric acid (GABA), serotonin, and acetylcholine (
45,
46). Other possible neural peptides having analgesia or antinociceptive properties are calcitonin, cholecystokinin, somatostatin, and neurotensin (
41).
Chronic Pain Theory
The chronic pain theory encompasses many of the physical, motivational, cognitive, and affective components of pain. The anatomic pain pathways are relatively clear and represent a mechanism for nociceptive pain in the animal model. Multiple pain mechanisms exist in the human model because of the complex integration of nociceptive stimuli, conceptual and judgmental factors, sociocultural influences, and the motivational and emotional states of the individual. Pain mechanisms include nociceptive pain, neuropathic pain, central pain, psychogenic, and operant pain. The human perception and reaction to pain are a blending of these mechanisms. The nociceptive pain mechanism is detailed in the pain pathways, as previously described, and represents pain originating from tissue damage, such as pain from cancer, degenerative joint disease, myofascial pain, and trauma. Neuropathic pain can originate from peripheral neurologic disease or injury. Central pain originates from denervation occurring after a cerebrovascular accident, spinal cord injury, or amputation. This pain may be due to a loss of the peripheral modulating influences on the central nervous system resulting in an unmodulated activity of afferent A-delta and C fibers.
Psychogenic pain is the interpretation of emotional distress as aversive and unpleasant sensation and its description in terms of pain language and behavior. The psychological states that are interpreted in this manner include anxiety, neurosis, hysteria, and depression. This mechanism of pain is often overlooked in patients with chronic pain.
Operant or learned pain behavior is often a major factor in chronic pain. Although the initial precipitating event causing the pain may be quite minor, the pain behavior is often long-lasting, owing to reinforcement by environmental influences. Pain behavior may be directly reinforced by family and physician attention or the delivery of medication. Indirect reinforcement (physical or psychological demands) occurs with avoidance of aversive consequences, which would have to be met if there were no pain. Operant pain behavior is also reinforced through a punishment cycle, when an injured party is overprotected and “punished” by outside factors if he or she begins to function more independently.
Genetics and Pain
The genetic determinants have been evaluated in humans with clinical pain (
47). The variability in pain syndromes has been associated with inherited genetic factors in back pain (
48), fibromyalgia (FM) (
49), menstrual pain (
50), and migraine (
51). Family and twin heritability estimates indicate genetic as well as significant environmental factors modulating pain (
52,
53). Polymorphic pain genes have been associated with
congenital insensitivity to pain (
54), drug metabolism due to cytochrome P450 (
55), familial hemiplegic migraine (
52,
56), FM (
57), and reflex sympathetic dystrophy (
58).
Gene therapy and other advances in molecular medicine may offer a means of enhancing antinociceptive receptors (cannaboid—1 and 2, acetylcholine—m and n, opioid—µ and κ, adrenergic—α
2) or blocking pronociceptive receptors (neurokinin—1-α-amino-3-hydroxy-5-methyl-4-isoazoleproprionate [AMPA], n-methyl-d-aspartate [NMDA]); acting directly on the calcium channels of pain fibers; or acting directly on membrane receptors, protein C-GAMA, or other areas of the central nervous system involved in the transmission of pain (
59,
60). Definitive applications of gene intervention in pain control remain to be developed.
Resolution of Pain
Acute pain is frequently the result of tissue damage in which the initial pain leads to an increase in anxiety, which magnifies the pain experience. The amount of anxiety generated and possibly the level of pain seem to be more influenced by the setting in which the pain develops rather than personality variables. With the healing process comes a reduction or termination of the anxiety and acute pain perception. When acute pain, which functions as a warning signal, fails to respond to treatment with conventional medical therapies, illness behavior and chronic pain develop. The anxiety characteristic of acute pain is replaced by depression with hopelessness, helplessness, and despair. When pain relief fails, physical activities decrease and suffering and depression increase.
Acute pain usually resolves when the source of nociception is removed or cured and resolves quickly with application of appropriate pharmacologic or regional analgesic therapy. The cause of acute pain can be documented by physical examination findings and diagnostic procedures. When indicated, appropriate operative intervention can be performed on the basis of these findings. A short course of analgesic medication usually controls postoperative pain, and a return to full, painless function can be anticipated in a matter of weeks. Acute pain control requires the administration of an efficacious analgesic dosage. Too little analgesia promotes suffering and anxiety, thus defeating the purpose of prescribing medications. Fear of drug addiction contributes to the underutilization of analgesic medications, and physicians tend to undermedicate in terms of frequency and dosage of pain medications (
61,
62). By prescribing low oral doses of opioids at infrequent intervals, physicians inadvertently force patients to adopt pain behavior in order to obtain adequate opioid analgesia. Pain behavior is characterized by high verbalization of pain, dependency, and the inability to work. Addiction in the acute pain situation is very rare, approximating less than 0.1% (
63,
64).
Unfortunately, a significant minority of acute pain patients continues to experience pain, which may progress into a more complex disease entity. Pain, a symptom of physiologic malfunction, now becomes the disease itself. Chronic pain represents a complex interaction of physical, psychological, and social factors in which the pain complaint is a socially acceptable manifestation of the disease. The etiology of chronic pain may be persistent nociceptive input, such as arthritis or terminal cancer; psychological disorders, such an anxiety, depression, and learned behavior; or social factors, such as job loss, divorce, and secondary gain.
The optimal treatment for chronic pain is prevention. Once the disease state of chronic pain commences, reinforcers such as monetary compensation, presence of job-related problems, manipulation of the environment to satisfy unmet needs, and retirement from the competitive world obstruct complete disease resolution. Therapies designed for acute pain are often contraindicated for chronic pain.
Prevention of chronic pain requires identifying contributing factors and resolving them early in the acute stage. Aspects worthy of attention include psychological stress, drug or alcohol abuse, and poor posture or muscle tone, as well as significant psychological and operant pain mechanisms. Physicians should set a reasonable timeframe for the resolution of the acute pain process. Patients should be advised when the pain medication will no longer be needed and that those medications that are no longer effective will not be continued. The patient’s attention should be directed to a gradual return of full activity on a prescribed schedule. Follow-up appointments should be planned at specified intervals so that the patient does not need to justify a visit. Work intolerance and job conflicts should be resolved.
Pain-Reinforcing Factors
Chronic pain syndrome is a learned behavior pattern reinforced by multiple factors. These behaviors are frequently found in individuals who are depressed, are inactive, and lack the skills or opportunity to compete in the community. These environmental factors promote pain behavior, regardless of the etiology of the pain, thereby distinguishing the patient with chronic pain from the population at large. Patients often develop a new self-image and see themselves as disabled by their pain. This self-perceived disability justifies their inactivity and manipulation of others and attempts to collect compensation. The typical patient often has been unemployed, has low job satisfaction, or has been on sick leave for long periods of time (
65,
66,
67,
68). Our data indicate that individuals who have been removed from the labor market because of pain for less than 6 months have a 90% chance of returning to full employment; those removed from the labor force because of pain for more than 1 year have less than a 10% chance of return to full employment (
66).
Individuals with chronic pain syndrome receive gains from their pain behavior; hence, they continue this behavior to maintain those positive reinforcers. Physicians reinforce the pain behaviors by lacking knowledge of this chronic disease process, failing to identify the chronic pain behavior and prolonging prescription of inappropriate medications, inactivity, and work limitations. The physician’s failure to acknowledge and direct the patient toward recovery tends to validate the chronic pain syndrome by providing an undiagnosable and untreatable problem. Family members also frequently reinforce the chronic pain behavior. They allow the individual
to become inactive and cater to the patient’s requests and needs over prolonged periods of time. In some instances, patients with chronic pain provide role models for pain or disability behavior for other family members (
69,
70).
DIAGNOSTIC AND CLINICAL EVALUATION
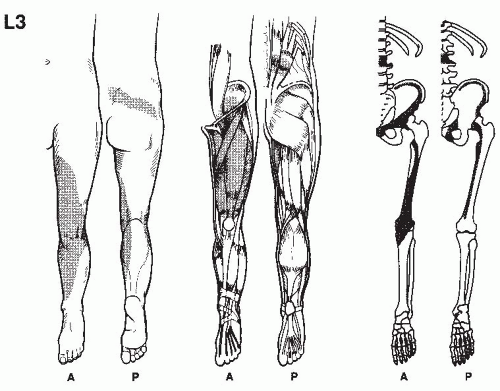
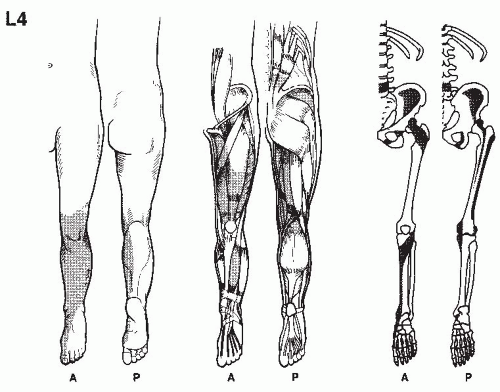
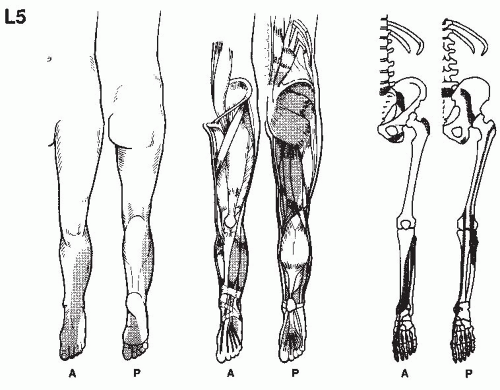
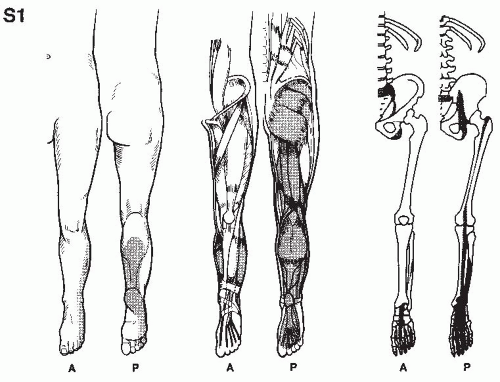
In establishing the etiology of pain, it is essential to consider its location, characteristics, its chronology, the limitations it imposes on the patient, and the results of previous therapy. This is accomplished by a thorough pain evaluation, including a detailed history, comprehensive physical examination, and review of appropriate diagnostic tests. When making the treatment plan, practitioners should keep in mind that many radiographic findings do not correlate with the patient’s clinical presentation, and that most physical exam findings can be nonspecific. Most pain patients present a complex array of physical, motivational, cognitive, and affective manifestations and therefore require detailed psychological and social evaluations. In the intensive effort to integrate these evaluations, it should be remembered that acute and ominous processes such as fractures, infections, and tumors must be ruled out, even if the patient has previously seen other providers.
History
A detailed history of the pain report identifies the pain in terms of its origin, radiation, quality, severity, and time intensity attributes, as well as mode of onset, duration, time of occurrence,
and factors that aggravate and relieve it. Previous treatments for pain should be noted, including comments regarding usefulness in the reduction of pain. Medications currently being taken, as well as those used in the past, should be recorded, along with the patient’s perceptions as to the results achieved by each. The details of physical therapy, including types of modalities, exercise, and effectiveness of regimes, should be recorded. An inquiry as to the patient’s attempts at biofeedback, relaxation, and hypnosis is also helpful. Information regarding associated findings including sensory deficits, muscle weakness, and altered body function should be obtained. It should be determined whether compensation is involved and if the patient is working; if not, the employment history should be obtained. The physical examination and related diagnostic studies should be directed toward evaluating the painful site and related regions. This process is useful in acquiring objective data to substantiate the clinical history.
Physical Examination
Physical examination begins when the patient is first seen and continues through every contact made with the patient. This provides the opportunity for the physician to evaluate how the pain affects motion and activities. Physical examination always includes examination of related spine and musculoskeletal components, as well as a neurologic evaluation. Painful regions need to be compared with normal areas on the contralateral side of the patient for sensation, temperature, and sensitivity to palpation.
Functional evaluation measures the appropriateness of the patient’s functional capabilities for the level of impairment. Objective, quantitative measurements give a baseline with which to evaluate progress and long-term outcome. Specific tools are available to describe and classify functioning, such as the International Classification of Functioning, Disability, and Health (ICF) (
113). The ICF characterizes the interaction between a health condition, personal attributes, and environmental influences. In this model, hierarchical levels categorize health-related states in increasing levels of detail to form a standardized description of the condition. Lists of categories relevant to specific diseases, known as ICF Core Sets, exist for different pain conditions including chronic widespread pain and low back pain (
114,
115,
116,
117,
118).
CLINICAL MANAGEMENT
The primary goals of treating a patient with pain are alleviating the pain and enhancing the patient’s quality of life and functional capabilities. The management of acute pain is based on pharmacologic, psychological, medical, and surgical innovations or advancements within the past century. And yet, the management of chronic pain has been recognized as a major health care problem only in the past 25 years. It remains unclear why some people become patients with chronic pain and others resolve their acute pain without significant difficulty.
Multidisciplinary Approach
The chronic pain problem is clearly multifaceted. No single physician has the resources to care comprehensively for the complex psychological, social, legal, medical, and physical problems involved in chronic pain. Therefore, the multidisciplinary team approach is necessary. Using a multidisciplinary approach does not mean the patient is referred from one specialist to another, because this tends to result in conflicting and overlapping treatment and a loss of hope of treatment in the patient. Ideally, the team should work together to provide a unified explanation of the illness and a comprehensive treatment program. The multidisciplinary pain service has the advantage of offering a variety of coherent treatment approaches to the patient. This type of program recognizes that a multifaceted problem requires a multifaceted approach, as well as continuity of care in which the patient is an active participant (
143).
The core group for the multidisciplinary treatment service should include a physiatrist, a pain medicine physician, a neurologist, a physiotherapist or occupational therapist, and a psychiatrist or clinical psychologist. This group may vary considerably according to local needs, resources, and available expertise. However, the team must have the knowledge to manage the psychological and social problems with optimal medical and anesthetic treatments. They must also have a thorough understanding of physical treatments and the rehabilitation process.
The multidisciplinary pain approach begins with a complete clinical evaluation. Comprehensive medical and psychosocial evaluations with particular emphasis on functional capabilities and behavioral responses to pain are essential. The somatic, affective, cognitive, and emotional components of the chronic pain experience are explored. All previous medical records are needed to avoid repeating appropriately performed studies and unsuccessful treatment approaches. This comprehensive clinical evaluation also includes functional capabilities to determine impairment level. The psychosocial evaluation focuses on the behavioral response to pain, adjustments to the physical impairment, and degree of motivation (
144). The MMPI-2 and other written tests are often used for generalized screening.
The multidisciplinary team functions at several levels within the treatment process. It attempts to identify and resolve documentable organic problems when present and to improve the patient’s ability to cope with the pain through medication, psychological intervention, and patient education. In addition, considerable effort is devoted to improving the patient’s functional outcome as measured by increased activity time, improved activities of daily living, increased distance walked, and increased tolerance for specific homemaking or vocational activities (
145,
146,
147). To accomplish these objectives, the multidisciplinary team must use many skills. In many cases, the patient with chronic pain is so entrenched in pain behavior that a behavior modification approach is essential. These patients are often characterized by low levels of activities of daily living, high demand for medication accompanied by physical and psychological dependency, high verbalization of pain, and the inability to work.
Pain Treatment Centers
The organization and operation of the multidisciplinary pain clinic has been discussed by multiple authors (
148,
149). Many behavior modification programs use the Fordyce model (
69,
88). This approach uses the general principles of interruption of the pain behavior reinforcement cycle, reward of healthy behavior, appropriate goals that the patient must achieve, measurement of improvement by functional assessment, as well as pain level, and psychosocial adjustment. Particular emphasis is placed on detoxification and medication reduction, pain reduction, increased activity, and modification of pain behavior.
If the patient is on pain medications and determined to be physically or psychologically dependent, he or she must be detoxified. This is routinely accomplished by establishing the equivalent dosage of each medication type (e.g., opioids, benzodiazepines, barbiturates, alcohol). Opioid medications are replaced with methadone, and long-acting barbiturates are replaced with phenobarbital or pentobarbital. Medication equivalents are placed in orange juice or in capsulated form and decreased at a rate of 5% to 10% per day. The medication is then given on an around-the-clock basis at fixed intervals. Gradual reduction of the pain ingredients occurs without significant side effects of withdrawal. The patient is not aware of the timing of the decrease but has been informed of the concept before starting the program. Nonsteroidal anti-inflammatory drugs (NSAIDs) and tricyclic or selective serotonin reuptake inhibitor (SSRI) antidepressants are routinely integrated as long-term medications. The pain management program is designed to reduce rather than eliminate pain, while increasing the patient’s functional capabilities.
The patient with chronic pain usually exhibits a decreased activity level, which results in a disuse syndrome. The exercise programs are based on the initial specific and general exercise that the individual can perform. The exercise regimen is progressive, with the goals rising along with the patient’s ability. Rewards for accomplishing tasks are a mainstay of this program with no reinforcement given for pain behavior. The achievable goals provide success and confidence and allow for frequent reinforcement when they are met. Cooperation by all staff members is essential; they must consistently ignore reports of pain and encourage improved function. Psychological intervention is used as indicated. The chronic pain behavior modification programs report short-term success rates in medication reduction, increased activity, and more productive behavior patterns (
150,
151). Statistics suggest improvement of 60% to 80% in patients with chronic pain without major psychosocial components, 30% to 50% in patients with significant psychosocial components, and approximately 20% in patients with major psychiatric components or secondary gains (
148,
152,
153,
154,
155,
156).
Multidisciplinary chronic pain treatment is a focused, unified approach to the chronic pain syndrome. In the United States, pain treatment centers differ widely in organization and emphasis. They are generally multidisciplinary centers that use some combination of anesthesiologists, clinical psychologists, dentists, neurologists, orthopedists, pharmacists, physiatrists, and psychiatrists. The goals of these centers are to diminish, if not eliminate, chronic pain; increase the patient’s functional capabilities to allow for a more active life; and decrease the patient’s dependence on drugs for pain control (
Table 49-4).
Physical Modalities
Physical modalities are valuable adjuncts in the successful management of acute and chronic pain. Therapeutic heat and cryotherapy are time-honored interventions in the treatment of painful musculoskeletal conditions. Clinical evaluations of transcutaneous electrical nerve stimulation (TENS), acupuncture, and cold laser have questioned the efficacy of these methods in alleviating discomfort (
157,
158,
159,
160,
161). For additional information on physical agents, the reader is referred to Chapter 63.
Pain arising from the musculoskeletal system is often caused by muscle spasm (
127). Heat and cold applications are primarily directed at reducing spasmodic muscle shortening. The shortened muscle may be a result of direct muscular trauma or underlying primary neurologic or skeletal disease. Investigators have studied the muscle spindle and its firing rate in relation to thermal changes (
162,
163). Direct and indirect effects on the muscle spindle are detected from both heat and cold applications (
164). The return of the muscle to its normal resting length is also believed to promote the reduction and resolution of pain (
165), but precisely how muscle spasm is relieved is not completely understood.
Physical modalities should be used with caution and to a limited extent. Passive treatment programs such as hot packs, massage, and ultrasound may be appropriate for a short period of time; however, an active treatment program should be implemented as early as possible. The patient should be transitioned to a home program involving exercise, stretching, and self-applied modalities as early as possible. Additional outcome studies have demonstrated the effectiveness of therapeutic exercise for chronic back pain patients. Unfortunately, there remains limited consensus regarding the most effective exercise programs for patients with low back pain (
166,
167,
168,
169).