paraspinal muscles (paramedian approach) or the interspinous ligament (midline approach), and ligamentum flavum, where increased resistance is usually felt. At this point, the needle stylet is removed and the epidural needle is connected, ideally via extension tubing, to a Luer-Lok low friction glass or plastic syringe filled with about 2 mL of preservative-free saline. (Although the syringe can alternatively be filled with air, this can theoretically lead to an air embolus with inadvertent intrathecal injection and is believed to cause a higher incidence of postepidural headaches.) As the operator’s one hand advances the needle slowly into the ligamentum flavum, the other hand exerts steady gentle pressure on the plunger of the syringe. Depending upon the experience of the injectionist and the patient’s body habitus, the entire procedure can either be done using an AP view, or additional lateral views can also be obtained to help judge the depth of penetration. Once the needle penetrates the ligamentum flavum, loss of resistance should be detected by the hand holding the Luer-Lok syringe because saline will be suddenly injected owing to the negative pressure within the epidural space. Aspiration is then performed to ensure no CSF or blood return. (If blood is present, the needle position should be readjusted until no blood return is found. If CSF return is present, the needle is either withdrawn and the procedure attempted at an adjacent level or a caudal or transforaminal approach considered for the epidural.) A small amount of contrast (usually in the range of up to several milliliters) is then injected to visualize an epidurogram pattern that can be described as a Christmas tree, a bunch of grapes, or a vacuolated pattern (Fig. 68-1). Two other contrast patterns are possible if there has been false loss of resistance (in which the needle has not yet penetrated into the epidural space) or accidental needle penetration through the subarachnoid membrane. In these cases, contrast pattern recognition is essential. For example, in situations of false loss of resistance, the injected contrast typically appears as a local accumulation of contrast, whereas a typical myelogram revealing a relatively tubular (column-shaped) contrast pattern is generated when there has been subarachnoid membrane penetration. In the latter situation, the needle should be withdrawn, and the injection can be reattempted at an adjacent interlaminar space or by switching to a caudal or transforaminal approach. Once the needle is confirmed in the epidural space and no vascular pattern is observed upon contrast injection, a mixture of 4 to 10 mL of solution containing 80 to 125 mg of preservative-free methylprednisolone or 12 mg of preservative-free betamethasone sodium phosphate (Celestone Soluspan) and preservative-free 1% lidocaine with or without saline is injected into the epidural space through the epidural needle.
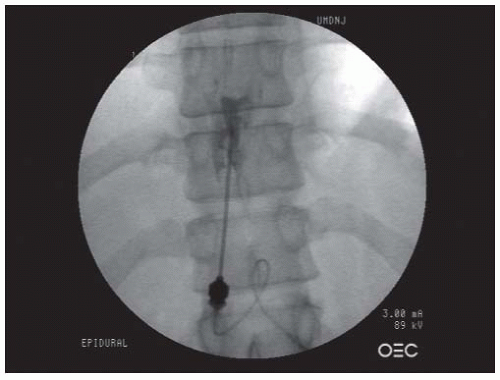
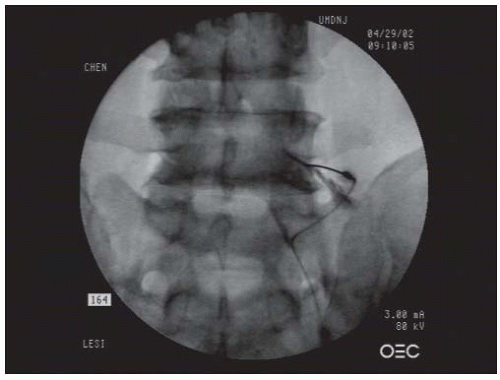
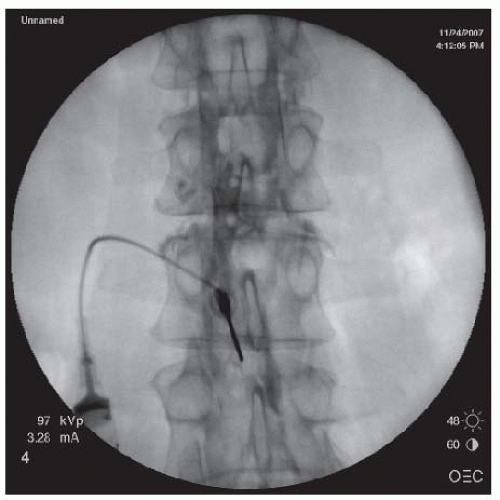 FIGURE 68-1. Lumbar interlaminar epidural injection. AP view showing a typical vacuolated epidurogram. |
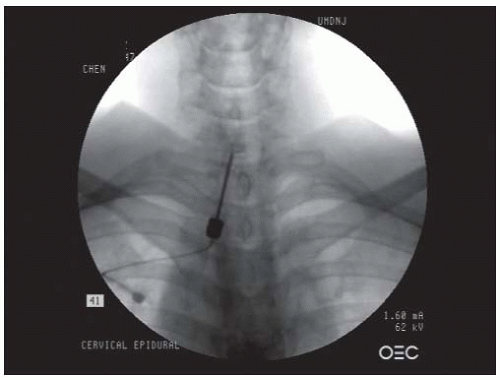 FIGURE 68-2. AP view of cervical interlaminar epidural injection demonstrating typical “honeycomb” pattern of epidurogram. |
region immediately under the pedicle, slightly lateral to the 6 o’clock position (Fig. 68-5). This position leads to needle placement in the neuroforamen, ventral to the nerve root. Lateral imaging is used to demonstrate the needle depth, which should be located at the superior portion of the intervertebral foramen, just under the pedicle (Fig. 68-6). An AP view is then obtained to ensure that the needle tip is located at the “safe triangle,” slightly lateral to the 6 o’clock position of the pedicle. The safe triangle is formed by the lower border of the pedicle, the lateral margin of the vertebral body, and the traversing nerve root. A needle position located within the safe triangle and lateral to the 6 o’clock position is deemed safe because it will not penetrate the nerve, blood vessels, or dura mater. Nevertheless, because of the precarious location of the nerve root and the DRG, caution should be exercised by advancing the needle slowly upon entering the neuroforamen, to avoid needle penetration of these neurologic structures. If the patient complains of radicular pain or paresthesias, the needle should be withdrawn and redirected superiorly. Once the needle is deemed at the proper position, approximately 1.0 mL of the contrast is injected under live fluoroscopic view. The needle should be redirected if there is vascular uptake of the contrast. The injected contrast should ideally outline the nerve root and also show epidural spread. Three milliliters of a mixture of solution containing 40 to 125 mg of preservative-free methylprednisolone, 6 to 9 mg of preservative-free betamethasone sodium phosphate, 40 to 50 preservative-free triamcinolone (33,34), or other equivalent dose of preservative-free corticosteroid and preservative-free 1% lidocaine can be slowly injected into the neuroforamen through the spinal needle (25,26).
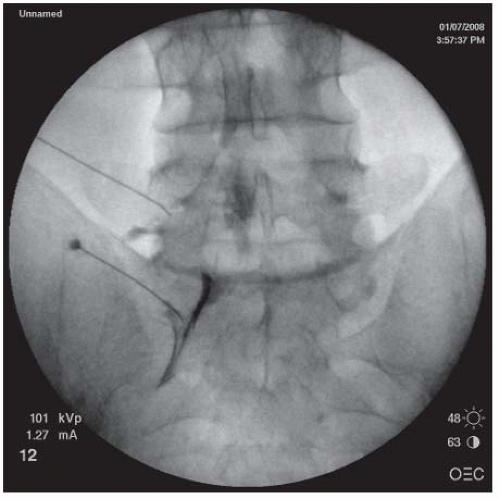
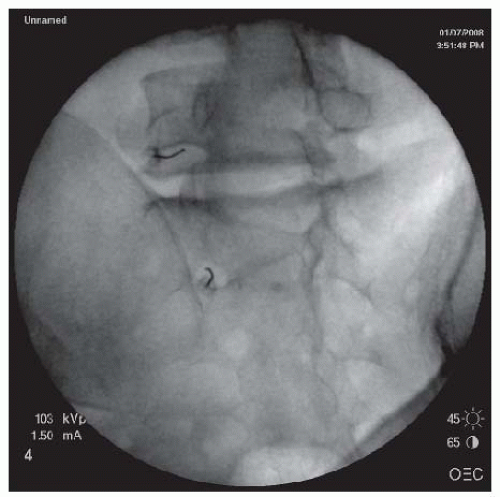 FIGURE 68-5. L5-S1 and S1 TEI. Oblique view showing needle just under the 6 o’clock position of the L5 pedicle and outer lateral quadrant of S1 for L5 and S1 TEIs respectively. |
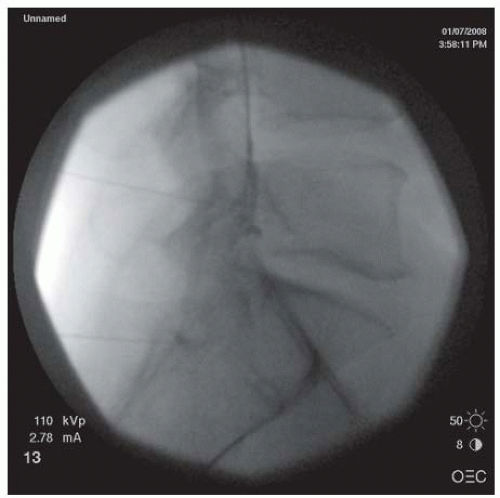
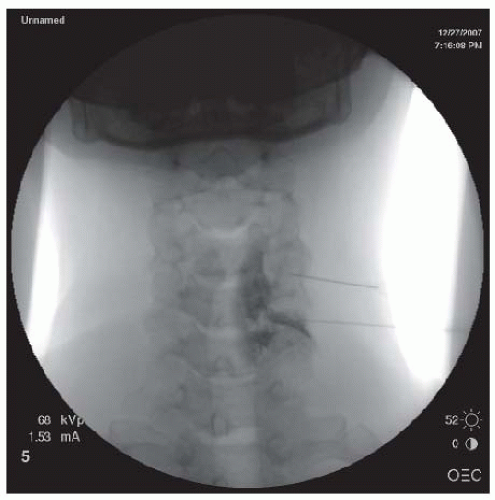
posterior wall of the neuroforamen. At this point, the needle tip is withdrawn and directed slightly anteriorly to “walk off ” the superior articular process and slip into the neuroforamen. The C-arm is turned to the AP view to assess the needle depth. The needle should be advanced in millimeter-by-millimeter increments in the AP view to ensure that the needle is not advanced past the center of the lateral mass (Fig. 68-8). Overzealous advancement of the needle into the inner half of the lateral mass can potentially lead to penetration of the dura into the subarachnoid space or into the spinal cord. The desired final needle location is the posterior wall of the targeted neuroforamen in the oblique view and the lateral half of the lateral mass in the AP view. After negative aspiration of the cerebrospinal fluid (CSF) or blood, 0.5 to 1 mL of contrast is injected under real-time imaging to exclude a vascular pattern. The needle should be repositioned if there is either blood flashback in the needle hub or a vascular pattern upon contrast injection. If the patient complains of paresthesias or radicular pain, the needle also needs to be repositioned. With satisfactory needle position, the injected nonionic water soluble contrast often outlines the exiting spinal nerve and fills the neuroforamen with epidural spreading or an epidurogram. After the satisfactory position, 1.0 mL of a test dose of 1% lidocaine is injected, and the patient is monitored for 2 minutes for any changes in vital signs or consciousness or neurological deficits in the extremities that would indicate an intravascular injection. For patients without abnormal signs, 40 mg of methylprednisolone, 6 mg of betamethasone sodium phosphate, or 10.25 mg of nonparticulate dexamethasone in a total volume of less than 2 mL per neuroforamen may then be injected (25,26,35). To prevent inadvertent arterial embolism into the spinal cord and brain stem, a nonparticulate soluble corticosteroid such as dexamethasone is recommended for cervical transforaminal ESI.
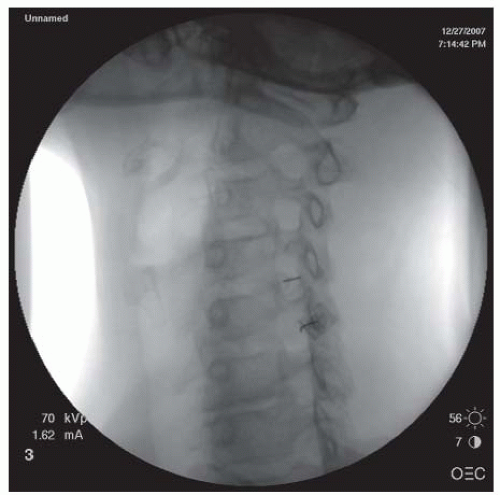 FIGURE 68-7. Oblique view of transforaminal cervical epidural injections showing needles in the posterior walls of the C4/5 and C5/6 neuroforamina. |
sacral hiatus. Alternatively, lateral imaging is used to view the bone defect, consistent with the opening of the sacral hiatus. The skin and the tissues overlying the sacral hiatus are anesthetized with 1% lidocaine. A 22- or 25-gauge spinal needle of appropriate length or a Tuohy epidural needle is inserted into the sacral hiatus. Loss of resistance can sometimes be felt upon needle penetration through the sacral ligament. Several milliliters of the contrast are injected in order to produce a sacral epidurogram to note the level that the contrast reaches. In the lateral view, a typical epidural contrast spread within the sacral canal resembles “smoke up a chimney” (Fig. 68-9), and in the AP view, it often looks like a “Christmas Tree” (Fig. 68-11). If a vascular pattern is observed, the needle should be withdrawn and redirected. Upon proper positioning, a mixture of 10 to 20 mL of solution containing 80 to 125 mg of preservative-free methylprednisolone or other equivalent doses of corticosteroid, preservative-free normal saline, and preservative-free 1% lidocaine is slowly injected into the epidural space through the spinal or epidural needle.
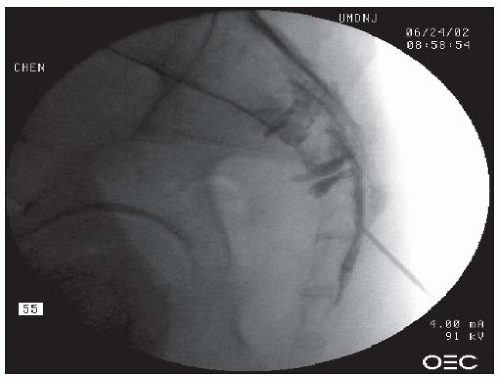 FIGURE 68-9. Caudal epidural injection. Needle tip in the sacral canal. Note the “smoke up the chimney” pattern of contrast in the epidural space of the sacral canal. |
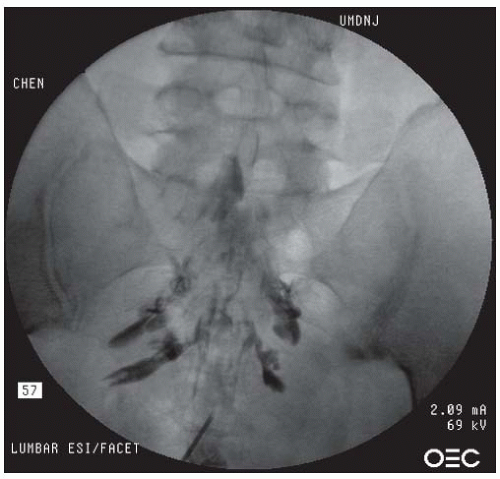 FIGURE 68-10. Caudal epidural injection. AP view demonstrating contrast in a “Christmas tree” pattern within the epidural space. |
given injection at the time of an intervening office visit before proceeding with another injection. Using this approach, the clinician can determine if another injection is still needed and can more readily alter their planned injection technique, rather than trying to make this assessment at the time of the scheduled injection itself.
TABLE 68.1 Volume of Injectate for Epidural Injections | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
TABLE 68.2 Dosage of Corticosteroids for Epidural Injections | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||
TABLE 68.3 Incorrect Needle Placement Associated with “Blind” ESI | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||
TABLE 68.4 Incidence of Intravascular Uptake (IVU) Associated with ESI | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||
stenosis. There is essentially no literature that correlates the type of disc herniation with the response of ESIs. It is the authors’ collective experience and observations that patients with large lumbar disc herniations obliterating the neuroforamen or extraforaminal herniations often have less benefit from ESIs. One study demonstrated that radiculopathy induced by the combination of spinal stenosis and disc herniation has less favorable outcome with ESI. In lumbar spinal stenosis, the efficacy of ESI correlated with the degrees and the levels of stenosis categorized by MRI (64). Patients with single-level lumbar spinal stenosis generally respond better than those with multilevel lumbar spinal stenosis. ESIs provide better efficacy in reducing pain and opioid consumption for patients with mild to moderate rather than severe stenosis. But a prospective cohort study with 12-month follow-up in patients with severe degenerative lumbar spinal stenosis found that fluoroscopic-guided and contrast-enhanced caudal ESIs reduced bilateral radicular pain and improved standing and walking tolerance (65). In contrast to radiculopathy due to herniated discs and/ or spinal stenosis, radiculopathy caused by epidural scar tissues or trauma such as nerve root stretch injury often responds poorly to ESI.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree