Chapter 25 The Musculoskeletal System and Bone Metabolism
Musculoskeletal manifestations involving the joints, muscle, bone, and supporting structures are common among patients with systemic lupus erythematosus (SLE) at diagnosis and throughout the course of illness.1–3 Although the pathomechanisms are less extensively described than for other lupus organ involvement and diseases like rheumatoid arthritis (RA), pain and fatigue are among the predominant health issues from the patients’ perspective.4
Arthritis
Arthritis is a dominant manifestation of active lupus. The 1971 American Rheumatism Association (ARA) preliminary criteria for the classification of SLE defined it as arthritis without deformity involving one or more peripheral joints characterized by pain on motion, tenderness, effusion, or periarticular soft tissue swelling. The 1982 revised criteria further increased specificity by defining it as nonerosive arthritis. In a subsequent comparison of the relative sensitivities of the 1971 and 1982 criteria in a cohort of patients with SLE, 88% met the preliminary criteria, and 83% met the revised criteria when arthritis was strictly classified as nonerosive arthritis. However, when arthritis was loosely defined as nondeforming arthritis without requiring radiographs, 91% met the revised criteria.5 These differences were not statistically significant, and variations in the sensitivities of the preliminary and revised definitions of arthritis when tested in various populations illustrate that in clinical practice, arthritis is a major though liberally defined feature of SLE.
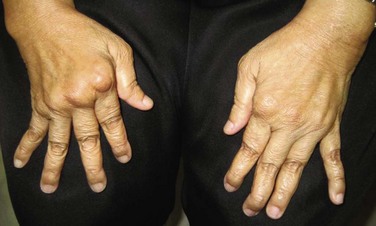
Erosions visible on radiographs develop in only a minority of cases, but joint space narrowing, subluxation, malalignment, and instability of joints often ensue even in the setting of relatively indolent arthritis. Studies of hand radiographs of patients with SLE and deforming arthropathy show only mild signs of bony pathology.6–8 Jaccoud’s arthropathy (JA), consisting of progressive rheumatoid-like deformities of the hands and feet, occurs in 3% to 43% of patients with lupus and can be clinically difficult to distinguish from RA, especially in the absence of extraarticular features (Figure 25-1). These joint deformities are usually due to a tenosynovitis rather than synovial hypertrophy. Histopathology reveals synovial membrane hyperplasia, microvascular changes, fibrin deposition, hematoxylin bodies, scant cellular infiltrates, and erosion of cartilage, but without the inflammatory pannus that plays a pivotal role in the cartilage and bone destruction in RA. Magnetic resonance imaging (MRI in a patient with JA demonstrates characteristic signs of soft tissue pathology, such as capsular swelling, edematous and proliferative tenosynovitis, synovial hypertrophy, and occasional bony alterations, for example, erosions, some of which are missed by conventional radiography.9–11 Rarely, erosive symmetric polyarthritis with deformities similar to those in RA, named rhupus, can occur in SLE and may represent a distinct lupus subset.7,12 In a study that classified patients with rhupus as fulfilling American College of Rheumatology (ACR) criteria for both SLE and RA, the presence of anti–cyclic citrullinated peptides (anti-CCPs) clearly distinguished patients with rhupus from those with lupus arthropathy whether deforming, nondeforming, or erosive. A strong association has further been observed between the presence of anti-CCP and the presence of erosive arthritis and major histocompatibility complex (MHC) class II alleles among patients with lupus.13
The management of arthritis includes background antimalarial drugs and glucocorticoids in appropriate doses for systemic flares. Nonsteroidal anti-inflammatory drugs (NSAIDs) should be used with caution in the presence of renal or cardiovascular involvement. The use of immunosuppressives and disease-modifying antirheumatic drugs like azathioprine, leflunomide, and cyclosporin to treat chronic arthritis is largely based on experience in RA. Methotrexate (MTX) is beneficial for the extrarenal involvement in lupus, having been shown to decrease overall disease activity and steroid requirement.14,15 The usual precautions apply, particularly the consideration of increased risk for MTX-induced adverse events in patients with renal impairment. Accelerated nodulosis induced by MTX similar to that seen in patients with RA has also been reported in patients with SLE and JA.16 Mycophenolate mofetil, proven effective for induction and maintenance therapy in lupus nephritis, has shown efficacy in ameliorating nonrenal manifestations of SLE17 and provides a suitable alternative in the treatment of lupus arthritis.
Despite the established efficacy of biologic agents directed against tumor necrosis factor (TNF) in RA and spondylopathies, their use in lupus arthritis has been restricted by reports of the development of autoantibodies and lupus-like syndromes.18,19 Nonetheless, an open-label experience with their use20 suggests that TNF blockade is effective in patients with SLE and arthritis, nephritis, and skin disease, and may be considered in lupus arthritis refractory to other therapies. Precautionary measures and vigilance must be exercised in monitoring for adverse events, including baseline screening and prophylaxis for infections like tuberculosis. Rituximab, a monoclonal antibody targeted against CD20 on B cells, has shown efficacy in RA but did not show any difference from placebo in a large clinical trial for active extrarenal lupus.21 Belimumab, which neutralizes B-lymphocyte stimulator (BLyS), is a newly approved biologic agent for SLE, having demonstrated benefit across organ systems including the musculoskeletal system.22,23 Other biologic agents, such as abatacept, which blocks T-lymphocyte co-stimulation, and the interleukin-6 (IL-6) receptor inhibitor tocilizumab have shown benefit in RA trials and are currently under clinical investigation for SLE.
Box 25-1 outlines the key features and general management of joint involvement in SLE.
Box 25-1
Joint Involvement in Systemic Lupus Erythematosus
• Arthritis is typically non-deforming and non-erosive in the majority of patients with SLE
• Jaccoud’s arthropathy (JA) consists of progressive rheumatoid-like deformities of hands and feet due to tenosynovitis rather than synovial inflammation and pannus formation
• “Rhupus” is characterized by erosive arthritis and a strong association with positive anti–cyclic citrullinated peptides (CCPs)
• Management includes analgesic and antiinflammatory medications. Disease-modifying antirheumatic drugs (DMARDs) and biologic agents may be useful in some cases.
Soft Tissue Disorders and Other Pain Syndromes
Patients with lupus may be at increased risk for localized soft tissue disorders owing to weakness, fatigue, and deconditioning, which occur with disease flares and long-term high-dose steroid treatment. There is a general laxity in connective tissue structures in patients with SLE,24,25 with anecdotal reports of spontaneous tendon ischemia, necrosis, and rupture associated with high-dose systemic corticosteroid therapy.26–28 Subcutaneous nodules are found in 5% to 12% of patients with SLE, generally in association with active disease. The nodules typically occur along the extensor surfaces of the upper extremities but may occasionally be found overlying the finger joints and along Achilles tendons. Although histologically similar to “rheumatoid” nodules, these nodules have no clear correlation with severe or erosive articular involvement and may be associated with MTX treatment.16,29
Generalized pain syndromes typified by fibromyalgia are particularly common in SLE, significantly contributing to poor quality of life. This topic is discussed in more detail in Chapter 52.
Muscle Involvement
Muscle pain, tenderness, and weakness are common manifestations during SLE disease exacerbations and usually reflect overall disease activity. On the other hand, inflammatory myopathy with muscle enzyme elevation or typical changes on muscle biopsy develops in 5% to 10% of patients and is indistinguishable from idiopathic inflammatory myopathy (IIM).30,31 In these patients, weakness, or less frequently, tenderness occurs primarily in the proximal limb-girdle muscle groups. Weakness is usually insidious in onset; patients experience easy fatigability manifesting as progressive difficulty in rising from a seated position or combing the hair. Most consistent with an active inflammatory myopathy is the elevation of serum muscle enzymes—creatine kinase (CK), aldolase, aminotransferases, and lactate dehydrogenase (LDH). The pattern of enzyme elevation varies among patients, making it necessary to measure all enzymes at baseline and serially monitor the abnormal levels to determine response to therapy. On the other hand, low creatine kinase levels may signal increased extramuscular active lupus.32
There is also a wide variation in electromyography (EMG) and muscle biopsy findings in SLE, depending on the population, selected test site, observer interpretation, and presence or absence of muscle symptoms. In patients with active symptomatic myositis, EMG demonstrates polyphasic motor unit potentials of small amplitude and short duration similar to those of IIM. Muscle biopsy is less sensitive in detecting muscle pathology among patients with SLE and is not routinely performed in clinical practice except in refractory cases or when other causes, such as drugs, need to be excluded. The findings have been described to vary from normal to interstitial inflammation, fibrillar necrosis, and degeneration. Immunopathologic staining studies further show evidence of vascular deposits of immunoglobulin, complement, or immune complexes in about a third of patients. Vacuolization and fibrosis are late occurrences and may signify irreversibility, although vacuolization may occasionally be found in reversible drug-induced myopathy.33–35 Among the histopathologic findings, lymphocytic vasculitis correlates with high erythrocyte sedimentation rates, arthritis, and Sjögren syndrome.36 In a study that compared clinical and laboratory features in 10 patients with SLE complicated by biopsy-proven myositis and those in 290 patients with SLE without myositis, those with myositis were more likely to have alopecia, oral ulcers, erosive joint disease, Sjögren syndrome, and presence of anti-ribonucleoprotein (RNP) autoantibodies, but less likely to have renal disease.37
The differential diagnoses in a patient with lupus presenting with muscle weakness include drug-induced myopathy (e.g., steroids, anti-malarials, statins38–40), concurrent endocrinopathies such as thyroid disease, and neurologic involvement such as chronic inflammatory demyelinating polyneuropathy.41 A thorough search for relevant clinical clues in combination with muscle enzyme measurements, judicious use of EMG, and muscle biopsy could prove useful in distinguishing inflammatory myopathy from these other conditions. Table 25-1 outlines the clinical features, pathomechanisms, and muscle biopsy findings in some causes of myopathy.
Musculoskeletal Infections
The range of musculoskeletal infections in SLE includes cellulitis, septic arthritis, osteomyelitis, pyomyositis, and other deep-seated soft tissue infections. The challenge posed by these infections lies in the difficulty of early recognition because the manifestations tend to be masked by immunosuppressive therapy, with tendency for involvement of multiple sites.42 Particularly difficult are chronic infections like those caused by mycobacteria that affect tendons, muscle, bone, and joints, sometimes mimicking or triggering active lupus disease.43 Although the management principles for these conditions are no different from those in the general population, the atypical presentation could cause undue delay in diagnosis and adversely affect outcomes. See Chapter 52 for a more detailed discussion on infections in SLE.
Avascular Necrosis of Bone
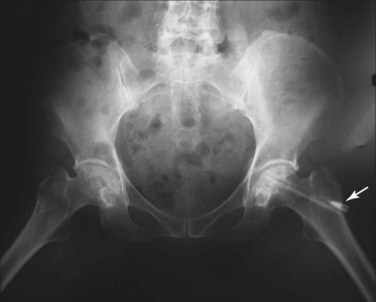
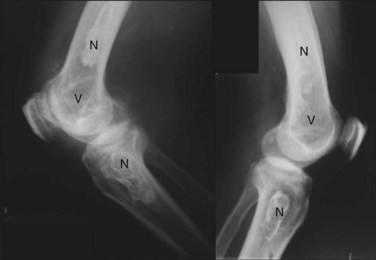
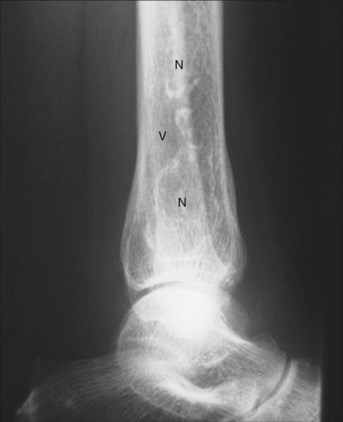
Avascular necrosis (AVN), also known as osteonecrosis, aseptic necrosis, or ischemic necrosis of bone, is reported in 5% to 30% of patients with SLE.44–52 It is a major source of morbidity especially among young patients with SLE. The terminology reflects its mainly vascular pathomechanisms, with the initial pathology described as interruption of the blood supply to the epiphysis, followed by reactive hyperemia and bone necrosis leading to subchondral fractures. Healing is characterized by new vessel formation and incongruous bony repair. With repeated microfractures and continued weight-bearing, the original fracture does not heal completely and new fractures occur, resulting in flattening of the surface and subsequent degenerative changes of the bone and adjacent structures (Figure 25-2).53,54 The epiphysis of the femoral head is particularly vulnerable to ischemic damage because of the undersupply of functional collateral end-arterial circulation.55 However, osteonecrosis can develop in other bones, including those at the knees, shoulders, wrists, and ankles, with a tendency to occur at multiple sites among patients with SLE.56–59 The lesions typically show on radiographs as bone infarcts characterized by serpiginous well-defined densities with sclerotic borders surrounding areas of bone necrosis (Figures 25-3 and 25-4).
Conditions associated with AVN include trauma, drugs, cigarette smoking, alcohol consumption, metabolic disorders, connective tissue disease, and organ transplantation. GC use is the most consistent risk factor for development of AVN in SLE.51,52,60–65 The pathomechanisms are postulated to be based on lipid-altering effects of GCs due to greater adipogenesis and fatty infiltration of osteocytes with increased apoptosis. The increases in femoral fat content and intracortical pressure compromise interosseous microcirculation, leading to bony necrosis.66–70 GC-induced AVN is usually dose related, with greater risk of AVN in patients receiving higher steroid doses, especially in the first year of treatment and with longer duration of therapy.63,64,71 The time interval between steroid use and the development of osteonecrosis varies among individuals, ranging from 1 to 16 months.71,72 Other contributory factors in the development of AVN in SLE are vasculitis, Raynaud phenomenon, cytotoxic treatment, production of inflammatory mediators, defects in fibrinolysis, gene polymorphisms, antiphospholipid syndrome, and other hypercoagulable states.64,73–79
The diagnosis of AVN should be considered in any patient with SLE who has persistent pain in one or a few joints even without evidence of disease activity in other systems, especially if GCs have been used as treatment. The pain is often insidious in onset and aggravated by weight-bearing and ambulation. In advanced disease, the pain is persistent even at rest. Limitation of range of motion that is not attributable to pain is usually a progressive and late symptom. The risk for development of osteonecrosis in the contralateral hip when one side is affected ranges from 31% to 55%.53
In a patient with suspected AVN, the diagnosis is confirmed by imaging studies. Plain radiographs can be completely normal in very early stages of AVN. In stage I of the widely used scale developed by Ficat and Arlet,80 routine radiographs are normal and the patient is usually asymptomatic or may experience only minimal pain. In stage II, radiographs show cystic or osteosclerotic lesions but no subchondral fracture. Stage III radiographs are characterized by the “crescent sign” resulting from structural collapse of a necrotic segment of subchondral trabecular bone; joint space remains intact. Stage IV represents end-stage disease and osteoarthritic changes are seen on radiographs. These last two stages connote irreversibility, with most patients remaining symptomatic and eventually requiring surgery.
The use of bone scintigraphy or technetium-labeled radionuclide bone scan in the early diagnosis of AVN is based on the increased osteoblastic activity and blood flow in the early stages of AVN. Computed tomography (CT) allows more detailed examination and can demonstrate the characteristic “asterisk sign” of a sclerotic rim surrounding a mottled area of osteolysis and sclerosis. Magnetic resonance imaging (MRI) has the greatest utility in the early diagnosis of AVN in a variety of anatomic locations. It is possible to detect bone marrow edema, an early feature of AVN, on MRI that is not visible on radiography or CT in early stages. Over time, the Ficat classification system has been modified by other groups to include these other imaging modalities and to assist therapeutic decisions. The Steinberg classification includes bone scan and MRI as well as volumetric assessment of the femoral head,81 and the Association of Research Circulation Osseous (ARCO) modification adds a stage 0 for patients in whom imaging findings are normal but who are at high risk for development of AVN.82
Early diagnosis is crucial to the successful treatment of AVN. The critical management decisions are whether to intervene surgically and which procedure to deploy. Conservative medical treatment options are advocated when the involvement is less than 15% of the articular margin and remote from the weight-bearing region.83 These measures are limited to load reduction on the affected region, such as the use of crutches, and physiotherapy to maintain muscle strength and prevent contractures. Unfortunately, these approaches do not generally prevent disease progression, and most patients eventually require surgical intervention.
Surgical management of femoral head AVN includes core decompression, structural bone grafting, vascularized fibula grafting, osteotomy, resurfacing arthroplasty, hemiarthroplasty, and total hip replacement.53,83 The timing and type of surgical intervention depends on the involved site and the stage of AVN, with little disagreement about the benefit of joint arthroplasty in stage III and IV AVN. The indications for surgery, including core decompression in the earlier stages, are controversial and based on the limited literature regarding the natural history of the disease. Among the identified risk factors for rapid progression of AVN, age younger than 40 years, abnormal lipid levels, and bilateral femoral head involvement identify patients who may benefit from early aggressive surgical intervention.84,85
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree