Chapter 128 The Infected Total Knee Replacement
Deep infection remains one of the most challenging complications following total knee replacement (TKR). The complexity and duration of treatment often impart dramatic physical, emotional, and financial costs for the patient and the treating physician(s).98 As the incidence rate of primary TKR continues to accelerate, it has been suggested that the demand for primary TKR is expected to grow by 673% in the year 2030.57 Although the incidence of deep infection after TKA remains relatively low, these workload projections suggest an associated increase in the number of infected knee arthroplasties requiring treatment.
In the face of an increasing prevalence of TKR, intensified efforts at infection prevention seem logical to reduce the overall burden of prosthetic joint infection (PJI). As an overview, prevention of PJI relies upon augmentation of the host response, optimization of the wound environment, and reduction of bacterial contamination in the preoperative, intraoperative, and postoperative time periods.41 In addition to these prevention efforts, a thorough understanding of the principles of proper diagnosis and treatment of the infected TKR is essential. Diagnostic and treatment principles for an infected TKR are often complex and require an individualized approach because of the multitude of variables affecting the final treatment outcome. These variables include the type of offending microorganism, the attendant host comorbidities, and the severity of damage to the local soft tissues and bone, as well as the level of expertise of available medical care.
In general, treatment of periprosthetic knee infection has become more standardized over the past several decades, primarily in terms of the approach toward routine use of high-dose local antibiotics and appropriate delay prior to reimplantation. However, indications for reinsertion of a new prosthesis after treatment for infection have liberalized significantly, so that the likelihood of successful treatment outcome or cure remains remarkably similar to that reported by early investigators, who used much more rigid patient selection criteria.50
Incidence, Risk Factors, and Prevention
Although the incidence rates of infection following TKR appear to have fallen over the past several decades, the reported incidence varies in many studies. Much of this variability is inherent in the relatively small numbers of patients studied and in the differences in patient populations described in these reports. As a result, the expected incidence varies widely according to risk factors present in the patient population and the expertise of providers being studied. For example, in 69,663 Medicare patients undergoing elective TKA, the incidence of PJI within 2 years was 1.55%, whereas the incidence occurring between 2 and 10 years was an additional 0.46%.59 In another report, with a different patient population, the rate of infected knee arthroplasties was 0.92%; in this study, urban nonteaching hospitals experienced the highest burden of infection (1.26%) compared with those in a rural setting (0.69%) and urban teaching hospitals (0.77%).58 Thus, any study reporting on the incidence of deep infection following TKR requires extensive stratification of patient risk factors and comorbidities, and an adequate description of the setting in which the procedures were performed.
Many inherent patient risk factors are known to predispose toward postoperative deep infection. Host factors include a diagnosis of rheumatoid arthritis,12,52,120 skin ulcers,120 diabetes mellitus,31,88 a history of malignancy,7 obesity,88 a history of smoking,88 renal or liver transplantation,103 HIV-positive status,87,109 prior open knee surgery or periarticular fracture,52 and prior septic arthritis or adjacent osteomyelitis.53 It is incumbent on the physician to be cognizant of these risk factors and to incorporate adequate screening measures and efforts to identify these risk factors into a disciplined preoperative evaluation process that facilitates optimization of host variables when possible.
Every effort should be made to incorporate differences in surgical technique and treatment algorithms that minimize the effects of these risk factors. For example, patients on immunosuppressive therapy should be optimized by altering or stopping their medications in the perioperative time period. A recent review summarized current recommendations for perioperative management of the more common antirheumatoid medications (Table 128-1).49 Other risk factors, potentially under the influence of the surgeon in the perioperative time period, that seem to predispose toward an increased incidence of deep infection include an increased international normalized ratio (INR) in the postoperative period,74 hematoma requiring reoperation,35 early wound healing complications,7,36 recent intra-articular injection of corticosteroid,85 and prolonged operative time.7,89
Table 128-1 Perioperative Antirheumatoid Medication Recommendations
| Medication | Important Drug Interactions | Comments |
|---|---|---|
| Corticosteroids | Corticosteroid use with fluoroquinolones increases the risk of tendon rupture. Antifungal agents and clarithromycin may increase levels of corticosteroids. | Perioperative use depends on the level of potential surgical stress. |
| Methotrexate | Methotrexate along with intravenous penicillins may lead to neutropenia. | Continue perioperatively for all procedures. Consider withholding 1 to 2 doses of methotrexate for patients with poorly controlled diabetes; the elderly; and those with liver, kidney, or lung disease who are undergoing moderate or intensive procedures. |
| Leflunomide | Leflunomide may elevate levels of warfarin and rifampin. | Continue for minor procedures. Withhold 1 to 2 days before moderate and intensive procedures, and restart 1 to 2 weeks later. |
| Sulfasalazine | May increase INR in patients on warfarin | Continue for all procedures. |
| Hydroxychloroquine | None | Continue for all procedures. |
| TNF antagonists | Avoid live vaccines in patients taking these agents; otherwise, no significant perioperative drug–drug interactions are known. | Continue for minor procedures. For moderate to intensive procedures, withhold etanercept for 1 week, and plan surgery for the end of the dosing interval for adalumimab and infliximab. Restart 10 to 14 days postoperatively. |
| IL-1 antagonist | None | Continue for minor procedures. Withhold 1 to 2 days before surgery and restart 10 days postoperatively for moderate to intensive procedures. |
IL, Interleukin; INR, international normalized ratio; TNF, tumor necrosis factor.
From Howe CR, Gardner GC, Kadel NJ: Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg 14:544–551, 2006.
Proper use of antibiotic prophylaxis represents the single most effective method of reducing infection in total joint arthroplasty.46 Optimization of the surgical environment with proper surgical protocols is beyond the scope of this chapter but is clearly under the influence of the surgeon and operating room personnel (Table 128-2).41,78 Use of low-dose antibiotic-loaded bone cement (ABLC) represents an additional surgeon-directed prevention measure for reduction of infection in high-risk patients and in revision surgery.52 Frequent irrigation, careful surgical technique, and excellent wound closure are important variables under the surgeon’s control.
Hematogenous infection of TKR in the early postoperative period, or many years after prosthetic replacement, is often influenced by the surgeon through education efforts made with arthroplasty patients. The rate of bacteremia after invasive procedures appears highest with oral procedures, followed by genitourinary manipulation, and lowest in association with gastrointestinal procedures.29 In general, invasive procedures that potentially cause bacteremia should simply be avoided in the first 3 to 6 months after TKR. In a retrospective review of late infections, seven cases were strongly linked to a dental procedure.115 Predisposing factors in these patients included risk factors such as rheumatoid arthritis or diabetes mellitus with dental procedure duration greater than 75 minutes.
Microbiology and Diagnosis
An appreciation of microbial pathophysiology is essential to the accurate diagnosis and subsequent treatment of infected TKR. As strange as it may seem, no criteria for the definitive diagnosis of PJI have attained universal agreement.82 Although many authors have suggested that positive cultures are required to make the diagnosis, it is well recognized that some true prosthetic infections are culture negative. In one report of 897 episodes of PJI, 60 (7%) occurred in patients for whom this was the initial episode of culture-negative results.9 The 5-year estimate of survival free of treatment failure was 94% for patients treated with two-stage exchange and 71% for patients treated with débridement and prosthetic retention. Culture-negative PJI has been reported to be as high as 19%.88
Our current definition of prosthetic infection includes a combination of clinical signs and symptoms, histologic analysis of tissue, and results of cultures. The diagnosis of definite infection is made if evaluation of the knee establishes at least one of the following criteria: (1) two or more cultures obtained by aspiration or deep tissue specimens obtained at surgery yield the same organism, (2) histopathologic evaluation of intra-articular tissue reveals changes in acute inflammation, (3) gross purulence is observed at the time of surgery, or (4) an actively discharging sinus tract is evident.43
It seems reasonable to assume that optimal treatment outcomes are achieved by accurately identifying the offending microorganism(s) whenever possible and enacting directed treatment strategies. Multiple reports have documented the distribution of microorganisms involved.* In most series, gram-positive organisms predominate; however, it is important to remember that polymicrobial infections represent approximately 9% of cases of infected TKR.88 It is also important to note that in the current era, many investigators are observing an increasing incidence of resistant organisms.86
Methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-resistant Staphylococcus epidermidis (MRSE) have emerged as common nosocomial pathogens often requiring complex antibiotics and potentially inferior treatment outcomes. In a study of 35 infected TKRs, those infected with methicillin-sensitive organisms demonstrated a treatment success rate of 89% compared with only 18% treatment success in those with methicillin-resistant infection.55 In contrast, a multicenter study of 37 patients with a resistant organism found only 24% with reinfection, with most of these reinfections caused by a different organism.75 These authors believe that two-stage reimplantation remains a viable treatment option for patients who have an infection with a resistant organism at the site of a TKR.
Periprosthetic fungal infections fortunately are rare, with Candida being the predominant species identified.91 A review of 10 cases documented successful treatment in 8 of 10 patients with appropriate antifungal therapy and two-stage reimplantation. Prosthetic joint infection due to Mycobacterium tuberculosis is also rare.8 To decrease the risk of reactivation of quiescent tuberculous infection, consideration should be given to preoperative or perioperative antituberculous prophylaxis.
Identification and diagnosis of biofilm organisms via traditional culture methods have lacked optimal sensitivity and specificity.107 Basically, several strategies can be used to address these organisms. Culture-independent molecular methods have been developed to improve the diagnosis of prosthetic joint infection in the research setting. Detection of 16S ribosomal deoxyribonucleic acid by polymerase chain reaction is just one example.69 One of the primary problems with this approach is the relative difficulty associated with excellent sensitivity of these techniques leading to false-positive test results.
An alternative strategy employs culturing of samples obtained from the prosthesis by sonication of explanted prostheses to dislodge adherent bacteria from the prosthesis.108 In this study, culture of samples obtained by sonication of prostheses was more sensitive than that of conventional periprosthetic tissue culture for the microbiologic diagnosis of prosthetic hip and knee infection, especially in patients who had received antimicrobial therapy within 14 days before surgery. This technique is now used in our institution as a matter of common clinical practice to improve the detection of organisms causing PJI.
Timing of the clinical presentation is a critical factor in the identification and implementation of the correct treatment strategy. These various clinical presentations have been characterized and classified as a useful guide to selecting the most appropriate treatment option for infected TKR (Table 128-3).99
Table 128-3 Classification System of Prosthetic Joint Infection: Time to Onset of Infection Dictates Treatment
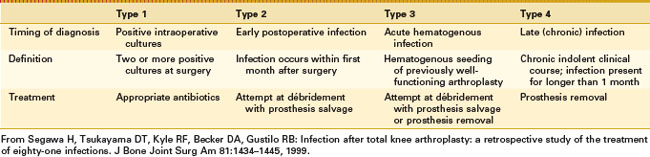
In this classification, postoperative infections diagnosed by positive intraoperative cultures after revision arthroplasty are generally low-virulence organisms such as coagulase-negative staphylococci and Propionibacterium spp.68 Five-year survival free of treatment failure for the 16 episodes was 89%. These results suggest a favorable outcome of prosthetic joint infections caused by low-virulence pathogens initially diagnosed as positive intraoperative cultures after revision arthroplasty.
For the remaining clinical presentations, rapid and expedient diagnosis is essential to prevent the delay of diagnosis, which could result in diagnosis of a late or chronic infection that could have been identified and treated as an early infection. Pain is the most common presenting symptom. Persistent wound drainage is strongly suggestive of infection and probably should be treated with arthrotomy, débridement, and irrigation within the first several weeks after surgery.117 Cultures of serous wound drainage are difficult to interpret and potentially misleading and therefore are discouraged. Empirical antibiotic use for persistent wound drainage should be avoided, as this only suppresses the clinical symptoms of infection and potentially delays diagnosis, eliminating the possibility of treatment of the infection without removal of the prosthesis.17,73,97,105
Diagnosis of early postoperative infection is typically confirmed by joint arthrocentesis, as the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels are nonspecific in the early postoperative period. CRP levels after TKR peak on postoperative day number two and decrease to preoperative baseline levels as early as 1 week but typically at 14 to 21 days postoperatively.11,60,118 In the early postoperative period, assertive management of delayed wound healing or marginal skin necrosis by débridement of necrotic skin and primary wound closure is preferable to empirical antibiotic treatment, prolonged observation, and eventual development of deep infection.62
An acute hematogenous infection typically presents with sudden onset of pain or stiffness in a previously well-functioning arthroplasty.5 Specific risk factors for a hematogenous infection, such as a remote source of infection or a recent invasive procedure causing significant bacteremia, should be identified.66 The severity of symptoms with pain, effusion, and restricted range of knee motion in this setting facilitates rapid diagnosis. Although ESR and CRP are typically elevated in these patients, the cornerstone of diagnosis is arthrocentesis with evaluation of the aspirate by gram stain, quantitative leukocyte count, and culture for aerobic and anaerobic bacteria. Empirical antibiotics for the unexplained painful prosthesis, without attempts at definitive diagnosis, unfortunately are commonly given; this approach only complicates subsequent efforts to diagnose deep infection.
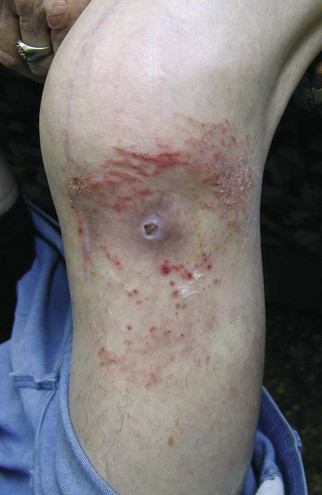
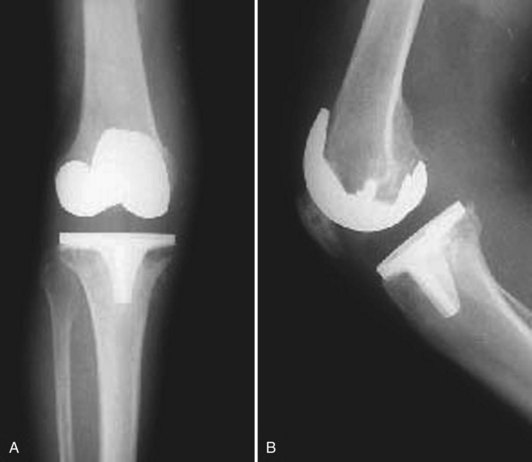
A vast majority of patients with an infected total knee arthroplasty (TKA) are diagnosed in the subacute or chronic setting. Historical factors such as persistent pain since the arthroplasty, prolonged postoperative wound drainage (Fig. 128-1), antibiotic treatment for difficulties with primary wound healing, and knee stiffness despite extensive rehabilitation efforts may be indicative of deep infection. Sequential comparison of plain radiographs may reveal progressive radiolucencies, focal osteopenia or osteolysis of subchondral bone, and periosteal new bone formation (Fig. 128-2).79 Additional studies should include CRP, ESR, and aspiration of the affected TKR. When elevated, CRP and ESR are obtained not only for diagnosis but to serve as baseline values for comparison with testing obtained during and after treatment.
In our opinion, arthrocentesis is considered an essential element of the workup and evaluation of a suspected deep periprosthetic infection. Synovial fluid analysis and culture enable identification of microorganisms, and knowledge of the synovial fluid cell count and differential provides an additional data point for diagnosis. Although these values may resemble a native joint infection in the acute hematogenous infection (neutrophil differential >90% and total nucleated cell counts >50,000/µL), it is important to note that most chronic TKR infections are associated with much lower values.37,70,106
Based on our experience, a synovial fluid leukocyte differential of >65% neutrophils (or a leukocyte count of >1.7 × 103/µL) is a sensitive and specific test for the diagnosis of prosthetic knee infection in patients without underlying inflammatory joint disease.106 Patients should have all antibiotics discontinued several weeks prior to aspiration, as this oversight frequently accounts for the inability to isolate organisms.4 Use of molecular genetic diagnostic methods with joint aspirates, such as the polymerase chain reaction technique, is potentially promising, but these remain experimental modalities for diagnosis of infected joint arthroplasty.24,69 The use of radioisotope scans to facilitate diagnosis of the chronically infected knee prosthesis is only occasionally useful.67,94,96
Despite all reasonable efforts to diagnose infection preoperatively, intraoperative evaluation of surgical tissue specimens may be necessary to confirm the diagnosis in difficult cases. The gram stain is notoriously unreliable, with a high percentage of false-negative results and an extremely low sensitivity.2,26 Frozen section testing to detect infection has been widely used with variable accuracy and results.25,33,64,80,83 Variability is likely accounted for by differences in technique, sampling errors during retrieval of tissue samples, pathologist experience, and the definition used to declare the presence of infection. Analysis of intraoperative frozen section is a reasonable and reliable method when an accomplished and experienced pathologist evaluates appropriate tissue samples.
Treatment Methods Whereby the Prosthesis Is Retained
Antibiotic Suppression
Antibiotic treatment alone will not eliminate deep periprosthetic infection but can be used as suppressive treatment when the following criteria are met: (1) prosthesis removal is not feasible (usually because of a medical condition that precludes an operative procedure), (2) the microorganism has low virulence, (3) the microorganism is susceptible to an oral antibiotic, (4) the antibiotic can be tolerated without serious toxicity, and (5) the prosthesis is not loose.112 The presence of other joint arthroplasties or a cardiac valvular prosthesis is a relatively strong contraindication to this treatment approach.
In a multicenter study, antibiotic suppression was successful in only 40 of 225 knees (18%).6 Combining several series reveals that antibiotic suppression was successful in 62 (24 %) of 261 knees.42 Use of a combined regimen of rifampin with a quinolone has been reported to be more successful than treatment with a single antibiotic.28 Despite the fact that most patients fail to meet all of these selection criteria, antibiotic suppression is commonly practiced, and this practice unfortunately prolongs the presence of infection and often complicates subsequent treatment attempts. Long-term antibiotic suppression should be initiated rarely and should be considered only when all treatment criteria are met.
Débridement With Prosthesis Retention
Open débridement may be indicated for the occasional acute infection in the early postoperative period (type II) or for acute hematogenous infection (type III) of a securely fixed and functional prosthesis. Suggested criteria for this treatment technique include (1) short duration of symptoms of infection (less than 2 weeks), (2) susceptible gram-positive organisms, (3) absence of prolonged postoperative drainage or a draining sinus tract, and (4) no prosthetic loosening or radiographic evidence of infection.18 Again, a relative contraindication for débridement and attempted salvage of the prosthesis is the presence of other joint replacements or of a cardiac valvular prosthesis.
Results of débridement are difficult to determine because of differences in microbiology and subsequent antibiotic management, variability in time to treatment, quality of the soft tissue envelope, extent and completeness of débridement, status of implant fixation, and the criteria for success in each report. A multicenter study reported a success rate of only 19.5% with open débridement of 154 knees.6 In another literature review, a success rate of 32.6% was reported in 530 infected TKRs treated with open débridement and component retention.101 Factors identified with failure of open débridement included postoperative drainage longer than 2 weeks in duration, existence of a sinus tract at débridement, hinged prostheses, and immunocompromised hosts.101
The importance of the timing of débridement in relationship to the onset of symptoms or the period of time elapsed since insertion of the prosthesis cannot be overemphasized.17,76,97,99,104 In a study of 24 infected TKRs treated with open débridement and component retention, a success rate of 100% was reported in the early postoperative infection group (type 2) and 71% in the acute hematogenous group with duration of symptoms less than 30 days.76 These authors emphasized the strict selection criteria, with patients demonstrating evidence of infection for less than 30 days.76 It is clear that débridement with prosthesis retention should not be attempted in patients with chronic infection.14,54,99
As just detailed, expeditious treatment provided as soon as possible after the diagnosis of infection has been established is a matter of paramount concern. This is particularly true for S. aureus, as delay beyond 48 hours after onset of symptoms resulted in a significant decrease in the success rate.18 The specific organism and its virulence are significant predictors of success following open débridement. It is well documented that S. aureus prosthetic joint infection is associated with the lowest success rate following débridement.23,97,120 Débridement attempts for resistant organisms are particularly unsuccessful, with a cure rate of only 18%.16
In contrast, in a report of 19 cases of infection with penicillin-susceptible streptococcal species, in which all surgical débridement procedures occurred within 10 days of symptom onset, treatment was successful in 89.5% of cases.73 In a recent report, only 1 (8%) of 13 patients infected with S. aureus was successfully treated, compared with 10 (56%) of 18 patients with S. epidermidis or a streptococcal species.23
Arthroscopy is not recommended as a method of débridement for the infected TKR, because the inability to perform satisfactory débridement of modular implants between the tibial tray and the polyethylene insert impairs success. In a series of 16 infected TKRs treated by arthroscopic débridement within the first 7 days after onset of symptoms, only 6 (38%) of the knees were successfully treated.114 These results were significantly worse than the 71% success rate obtained with open débridement of 24 TKRs, also performed by these authors.76 One of the primary reasons for recommending against arthroscopic débridement is that once arthroscopy is performed and subsequent antibiotics are started, failure of treatment with this approach extends the timeline, so that failure occurs long enough after the onset of symptoms that prosthesis removal is required.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree