CHAPTER 93 Syringomyelia
Syringomyelia, or cavitation within the substance of the spinal cord without an ependymal lining, has been recognized for more than 300 years as a pathologic entity. Etienne is credited for the first pathologic description in 1564 in La Dissection du Corps Humain; he described a cystic lesion in the spinal cord that contained a “fluid, reddish, like the fluidity of that of the ventricles.”1
Portal, in 1804, first appreciated and connected the clinical syndrome of an intramedullary cyst with the pathologic changes of the spinal cord.2 Ollivier then coined the term “syringomyelia,” combining the Greek words for “tube or pipe” and “marrow.” He documented a connection between the fourth ventricle and this cystic structure, which he believed to be a congenital anomaly.1,3,4
Those ependymal-lined cavities that appeared to be pathologic dilatations of the central canal were termed hydromyelia. Some authors viewed hydromyelia, in which the central canal was dilated but preserved, and syringomyelia, with or without a connection to the central canal, as stages of a common process. Hence unification of the terms resulted in the concept of syringohydromyelia or hydrosyringomyelia.1–35
In 1973 Barnett published the first English-language monograph on syringomyelia.6 He proposed a classification based on a variety of clinical and experimental observations and studies. The classification scheme consisted of two broad categories: (1) communicating syringomyelia (e.g., Chiari I malformation, Chiari II malformation, basilar arachnoiditis) and (2) noncommunicating syringomyelia (e.g., occurring with spinal dysraphism, spinal cord trauma, spinal cord tumor, spinal arachnoiditis) (Box 93–1).
Recent experimental and clinical work including that of Oldfield and Milhorat and their colleagues7–13 has helped to clarify the pathophysiology and treatment of this complex syndrome.
Etiology, Pathology, Pathophysiology, Prominent Theories
Historical Perspective—Early Theories of Syringomyelia
Although the pathogenesis of syringomyelia has not yet been completely defined, the association between syringomyelia and congenital abnormalities was appreciated long ago. Tamaki and Lubin14 credited Baulmer of establishing this relationship in 1887. Poser noted that Schlesinger’s 1895 monograph stated that there was an associated congenital abnormality in one third of the cases of syringomyelia that he had reviewed.15
Ollivier d’ Angers formulated and Leyden further refined the developmental theory of syringomyelia formation.15,16 These authors stated that syringomyelia must be considered a congenital disorder associated with embryonic maldevelopment, specifically incomplete occlusion of the primitive fold. This improper fusion of the two folds of the primitive medullary groove allowed the abnormal lining of germinal cells to persist, resulting in simple hydromyelia.
Another theory from the congenital viewpoint was that there was a problem inherent in the environment to which the fetus was exposed. Kahler and Pick16 in 1879 theorized that chronic intrauterine inflammation resulted in gliosis and aberrant development of the spinal cord that subsequently led to syrinx formation. In 1910 Haener,16 on the other hand, proposed that events during the act of birth (e.g., trauma) may arouse neural activity in abnormally enclosed tissue, with resultant syrinx formation.
W.J. Gardner—Hydrodynamic Theory “Water Hammer”
In a series of landmark papers, W.J. Gardner expounded his hydrodynamic theory of the pathogenesis of syringomyelia.17–23 Gardner’s theory was the first among three prominent theories to survive up to modern clinical practice. He based his theory on three observations: (1) dye injected into the ventricular system was recovered from the syrinx at operation, (2) fluid withdrawn from the syrinx at operation strongly resembled cerebrospinal fluid (CSF) found in the ventricular system, and (3) experimental hydrocephalus produced by obstruction of the normal outflow of CSF from the fourth ventricle resulted in the formation of syringomyelia that was in communication with the ventricular system.24
The pulse wave effect of the diverted CSF acted as a water hammer, gradually dilating the central canal or dissecting the substance of the spinal cord around the canal and creating a syrinx. From the perspective of Gardner, a congenital hindbrain defect that obstructed the CSF flow from the fourth ventricle to the subarachnoid space was the sine qua non of syringomyelia. Ball and Dayan25 and West and Williams26 questioned Gardner’s theory and the necessity of a direct communication to the fourth ventricle for production of a syrinx. To support this statement, Milhorat and colleagues7,27 demonstrated in large autopsy studies that the majority of syrinxes did not communicate with the fourth ventricle and that the central canal was not patent in most normal adult patients.
B. Williams—Craniospinal Pressure Dissociation Theory
Williams also believed that the cavity enlarged after its initial formation as the result of compression of the lower end of the cavity with the rapid filling of the epidural venous plexus during a cough or sneeze. The fluid in the syrinx was then propelled rostrally, dissecting the central canal or pericentral parenchyma of the spinal cord. Williams applied the term “slosh” to this part of his theory to explain syrinx extension.28
E. Oldfield—Abnormal Pulse Wave Theory
Oldfield and colleagues13 used magnetic resonance imaging (MRI) with and without cardiac gating, intraoperative ultrasonography, and direct intraoperative observation of the exposed hindbrain and documented the downward movement of the cerebellar tonsils during systole. This group interpreted the data as obviating the necessity of a direct communication with the fourth ventricle, as advocated by Gardner. Moreover, they observed that the syringomyelic cord did not enlarge with Valsalva maneuver and that venous pressure had little to do with syrinx elongation, disputing the Williams “suck and slosh” theory. The authors proposed that the abnormal pulse wave in the spinal subarachnoid space, caused by the partial obstruction by the hindbrain, placed pressure on the spinal cord and dissected the central canal, causing the cyst to enlarge.
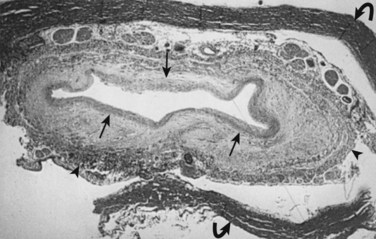
Milhorat and colleagues12 proposed that normal CSF flow was from the spinal subarachnoid space through the parenchyma of the spinal cord into the central canal. The CSF then flowed into the fourth ventricle outlet at the obex. Their theory was supported with a rodent model of syringomyelia. They injected kaolin into the central canal of rats, causing stenosis of the proximal central canal through an inflammatory reaction. A resultant syrinx was formed. The authors suggested that syrinx formation was due to disruption of normal CSF flow by the inflammatory stenosis (Fig. 93–1).
Communicating Syringomyelia
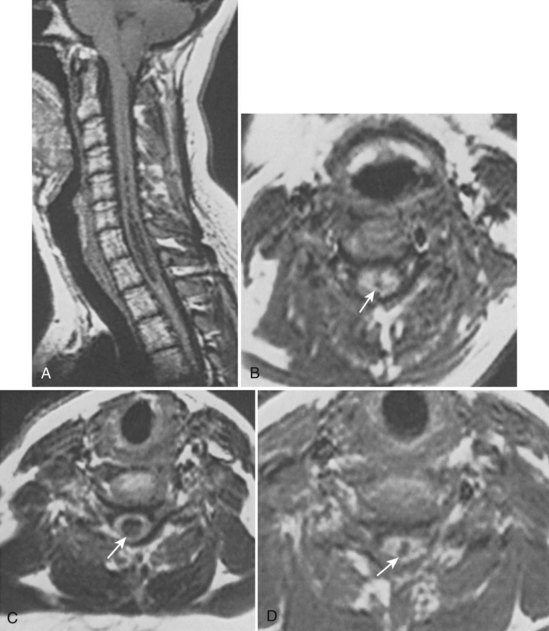
In 1896 Chiari published an addendum to an earlier work in which he described anomalies associated with hydrocephalus. In this latter publication, there were descriptions of patients with hydromyelia. Gardner and Goodall found that a majority of patients undergoing surgical decompression for symptomatic Chiari I malformation had a concurrent syringomyelia (Fig. 93–2).29 Gardner and colleagues30 demonstrated, at operation, communication between the syrinx of the upper cervical cord and the ventricles in patients undergoing suboccipital craniectomy and cervical laminectomy for decompression. Indigo-carmine was injected into the patient’s lateral ventricle, and colored CSF was recovered by direct puncture of the cervical syrinx.
Appleby and colleagues31 established that a “communicating” type of syringomyelia could also be acquired from chronic arachnoiditis involving the basal cisterns and obstructing the outflow of CSF from the fourth ventricle.
Noncommunicating Syringomyelia
Syringomyelia Associated with Spinal Arachnoiditis
The association between spinal arachnoiditis and syringomyelia was first reported by Vulpian in 1861 and by Charcot and Joffroy in 1869. Some authors believed occlusion of blood vessels supplying the cord from profound arachnoid scarring was the underlying pathophysiologic process for intramedullary cavitation.32–34
As previously described, Williams implicated craniospinal pressure dissociation, secondary to obstruction of the subarachnoid space, as the factor responsible for cyst formation and extension in this disease entity.35 In 2004 Chang and colleagues36 explained that the blockage of the spinal subarachnoid CSF pathway produces a relative pressure gradient inside the spinal cord distal to the blockage point that induces CSF leakage into the spinal parenchyma and the formation of syringomyelia. Barnett37 and Milhorat7 considered this type of syringomyelia to be of the “noncommunicating” type because no connection between the cyst and fourth ventricle could be demonstrated., Koyanagi and colleagues38 reviewed a series of 15 patients who underwent various shunting procedures for syrinx treatment caused by spinal arachnoiditis. Although neurologic improvement was found in a decent percentage of patients (60%), many required additional shunting procedures over time due to catheter blockage or failure.
Syringomyelia Associated with Spinal Cord Tumors
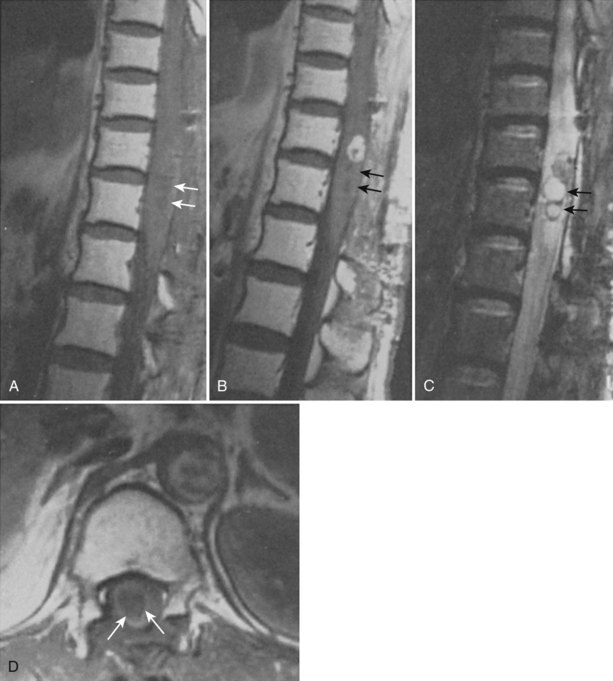
The association between syringomyelia and spinal cord tumors has been well established. Simon in 1875 was the first to report the simultaneous occurrence of syringomyelia and spinal cord tumors.39 Proposed mechanisms for syrinx formation in this environment include (1) edema, (2) blockage of the perivascular spaces with resultant tissue fluid stasis, (3) cavitation secondary to disturbance of blood supply to the spinal cord, and (4) spontaneous hemorrhage or autolysis of the mass.33,40,41 Other authors contend that syringomyelia associated with a spinal cord tumor is due to a direct effect of the neoplasm.15,39,42–44 Some authors believed that this disordered gliosis observed with tumor presence was also the underlying pathophysiologic cause even in cases not associated with an intramedullary neoplasm; this led some physicians to recommend radiation therapy as a rational but extreme form of primary therapy.1,45–48 Today, the use of radiation therapy should be restricted only for primary therapy of a known neoplasm or as an adjunct to surgical resection of tumor (Fig. 93–3).
Spinal CSF Dynamics in the Presence of a Neoplasm
Total or subtotal obstruction of CSF flow by an intramedullary or sometimes extramedullary/intradural tumors may be a significant factor for the development of syringomyelia. The subarachnoid and the extracellular space of the central nervous system should be considered as a single-fluid compartment with no barrier to fluid movement between them. Interference with this normal CSF flow influences extracellular fluid flow out of the spinal cord. The high ratio of syrinx cavities associated with intramedullary tumors may be attributable to their simultaneous influence on the subarachnoid and extracellular spaces. Fluid transudation from tumor vessels, breakdown products of tumor cells, and in some cases active secretion also raise the protein content and thus the viscosity of the extracellular fluid contributing to further aberrances on normal flow dynamics.49 Less commonly, intramedullary cysts can present in association with an extramedullary neurofibroma or meningioma. The removal of the extramedullary mass lesion is usually followed by a spontaneous collapse of the associated syrinx.
Syringomyelia and Spinal Cord Tumors—Clinical Studies
In a surgical series of 100 intramedullary tumors, 45% presented with associated syringes.49 A syrinx was more likely to be found above than below the tumor level. Ependymomas and hemangioblastomas were the most common tumor types to be associated with syringes. Astrocytomas, on the other hand, tended to demonstrate syringes less often. The higher the spinal level, the more likely a syrinx was encountered.
Syringomyelia Associated with Spinal Cord Trauma
The pathophysiologic basis for the formation and extension of syrinxes of the injured spinal cord remains the subject of considerable debate. However, a constant factor in all cases is the alteration of normal CSF flow dynamics. The onset of signs and symptoms of progressive post-traumatic cystic myelopathy ranges from as early as 2 to 3 months following injury to as long as 30 years after injury.50 Approximately 4% to 10% of patients suffering a traumatic spinal cord injury develop progressive spinal cord dysfunction associated with an expanding syrinx.23,51,52
Post-traumatic Syringomyelia—Human Studies
Milhorat’s large autopsy study demonstrated that syrinxes associated with trauma had distinctly different histopathologic findings and were associated with different clinical symptoms when compared with those lesions that were in communication with the fourth ventricle or those cavities that appeared to be isolated dilatations of the central canal.7 These syringes involved the parenchyma of the cord asymmetrically, were not associated with the central canal, and often extended to the pial surface. Examination of pathologic specimens revealed nonreversible damage to spinal nuclei and tracts including focal necrosis, central chromatolysis, and wallerian degeneration.
Post-traumatic Syringomyelia—Mechanisms of Development
The possible factors implicated in the production of the initial cystic lesions in post-traumatic spinal cords included ischemia secondary to arterial and/or venous obstruction, tissue breakdown from lysosomes or other intracellular enzymes, liquefaction of a prior hematoma, mechanical damage from compression of the substance of the cord at the time of initial injury, or tethering by delayed formation of extensive subarachnoid adhesions and/or a bony gibbus.53–61
Similarly, the mechanism for the extension of the syringomyelia remains a matter of controversy leading to several mechanisms being proposed. Some view the rostral and caudal extension of the syrinx, which produces late neurologic symptoms, being a result of a one-way valvelike trapping effect of the subarachnoid space into the cavity.11,62–66 Less frequently observed presentations of post-traumatic cysts include patients with a history of a herniated cervical or thoracic disc with resultant ventral compression from a bony or soft gibbus and disturbance of normal CSF flow. Spinal puncture with injection of an irritative dye (e.g., methylene blue) can cause an ascending arachnoiditis that may develop associated subarachnoid and/or intramedullary cysts. Patients who have subarachnoid hemorrhage may also develop spinal cord tethering with associated intramedullary or subarachnoid cysts.
One author has suggested that post-traumatic syringomyelia may be classified into two types. These authors contend that successful reestablishment of normal CSF flow dynamics by untethering the spinal cord stops the progression of clinical decline and in some cases may return valuable lost function. A preoperative distinction could be made on the basis of the presence (high-pressure type) or absence (low-pressure type) of the flow-void sign on a T2-weighted magnetic resonance image. With a midline myelotomy, fluid within a high-pressure syrinx would pour out, resulting in sustained neurologic improvement. In the low-pressure type, drainage of the syrinx would not collapse the expanded spinal cord and the surgical outcome would be modest at best.67
Clinical Features
Hydrocephalus may be found in 10% to 33% of patients but is more likely related to an associated Chiari malformation.26 This frequent association supports the concept of a hydrodynamic mechanism for the formation of syringomyelia.
Syringomyelia—Physical Examination
The autonomic pathways of the interomediolateral column may be affected, resulting in Horner syndrome, trophic changes of the skin, a neurogenic bladder, and dyshidrosis. Corticospinal tract involvement may give spastic paraparesis. Patients experience a loss of deep tendon reflexes in the upper extremities. A skeletal survey may reveal congenital anomalies including basilar impression and invagination, Klippel-Feil deformity, and spina bifida occurring primarily at C1.68 Developmental scoliosis may occur as a result of cord cavitation and is probably not an unassociated congenital lesion.69 Although discussed extensively in the literature, painless joint destruction (Charcot joints) occur in less than 5% of patients with syringomyelia.70
The clinical course of syringomyelia is usually insidious, worsening over several years to decades. Alternatively, the patient may worsen in an abrupt stepwise fashion punctuated by intervals of clinical stability.71 The prognosis of untreated syringomyelia is compatible with modified but productive survival for decades in 50% of surviving patients.72 The remainder become incapacitated and die as a direct result of the pathologic process. Seki and Fehlings73 designed a rodent model to further elucidate the role of post-traumatic syringomyelia in spinal cord injury.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree