CHAPTER 108 Surgical Procedures for the Control of Chronic Pain
The results of surgical therapy for chronic benign pain syndromes are poor.1,2 Surgical experience with central deafferentation (e.g., cordotomy)3–9 suggested that patients with neoplastic pain achieved satisfactory results more frequently than those with benign pain. Apparently, the longer life expectancy of patients with benign pain allowed neural plasticity to overcome surgical interruption.10 This was borne out in studies of cordotomy or dorsal rhizotomy.11–17 A distinct characteristic of refractory benign pain is that it appears to follow lesions of the nervous system. This is in contrast to malignant pain, which is thought to be nociceptive.
Mechanisms of Chronic Pain Production
The immediate effect of injury is to activate receptors to cause firing in specific nerve fiber types. These include large myelinated Aβ fibers, small myelinated Aδ fibers, and small unmyelinated C fibers. The interaction of these afferents, through the substantia gelatinosa, forms the basis for the gate control theory of pain. The cell bodies of primary nociceptive neurons are located in the dorsal root ganglion, with afferent synapses in layer I or V. Layer I cells are nociceptor specific and somewhat less discriminatory. Layer V cells respond to many inputs, mainly repetitive nociceptive stimuli and nociceptive input. The primary nociceptive neurons synapse on rostrally projecting second-order neurons in the dorsal horn, the theoretic target of the “gate.”18 After postinjury discharge, the next normal event would be for the fibers to return to rest. In the face of persisting injury, however, repeated firings are provoked; some receptors become more sensitive to subsequent stimulation and can, in fact, fire without further stimulation. This sensitization can arise from direct change in the structure of the nociceptor13,19,20 or as a response to substances released in its milieu.21–24 As damaged tissue heals with scar formation, granulation tissue containing nerve sprouts and capillaries invades the area. This further changes the local environment and the properties of the nerve endings. Such changes have relevance in the healing of surgical wounds such as those from laminectomy or spinal fusion; during healing, there may be significant pain problems generated by the wound itself.
The physiologic responses to nerve damage are complex and variable. Devor broadly classifies these into three groups.25 The first, sensitization of the nociceptor endings, is characterized by a reduced activation threshold for activation; in such a state, non-noxious stimuli may become capable of producing pain. Second, the nociceptive fibers themselves become a source of pain when they are activated at abnormal locations along their course; this is the phenomenon of ectopic electrogenesis. Third, pain could result from abnormal central processing of afferent impulses. In the setting of chronic spinal pain syndromes, the first two possibilities are most pertinent, with true central pain syndromes being uncommon.
Chemical21,22,24 and mechanical26–28 stimuli can invoke or modify repetitive discharge in the damaged nerve. Epinephrine and norepinephrine can both activate afferent fiber endings in neuromas; these responses are thought to be mediated by α-adrenergic receptors.23,29,30 Experimental observation indicates that sympathetic system activity can produce abnormal sensation through neural transmitter release that stimulates afferent nerve sprouts possessing ectopic adrenergic chemosensitivity.30 The abnormal sensations produced by these mechanisms may explain causalgia and other sympathetic dystrophies,31,32 along with the potential benefit of sympathectomy for such disorders.
The phenomenon of ectopic electrogenesis, which occurs in neuromas, can also develop in axons that have become demyelinated but remain in continuity. This issue relates directly to chronic low back and leg pain, in which a neuroma would not be expected to form, but in which demyelination of nerve roots is a known complication. Such demyelinated roots may exhibit either hyposensitivity or hypersensitivity. Spontaneous discharge has been shown to occur at sites of peripheral demyelination.32 These discharge patterns are similar to those found in neuromas.25 Hence nerves with regions of demyelination can demonstrate ectopic electrogenesis, which transfers nociceptor-like information into the central nervous system. Rhythmic firing, a characteristic of cell behavior not elicited until a certain threshold level of generation current is reached,33 can be provoked in demyelinated regions by mechanical stimuli. This threshold characteristic is important because many injured nerves appear to be poised near the rhythmic firing threshold. Hence brief or weakened stimuli can set off prolonged discharge that may persist beyond removal of the stimulus.25 In experimental preparations, tetanic stimulation produces this so-called after-discharge, which is followed by a period of prolonged electrical silence.34 It is evident that this could have implications in pain relief from spinal cord stimulation.
The dorsal root ganglion demonstrates mechanical sensitivity in its normal state and has such a high level of baseline excitability that some discharge occurs spontaneously35; after discharge occurs, stimulation is common. This baseline excitability is heightened after peripheral nerve injury. In this instance, the dorsal root ganglion contributes ectopic barrages above and beyond those generated by the region of peripheral injury.36,37 The state of excitability of the dorsal root ganglion is thus of clinical importance in root compromise.32 In the chronically injured root, deafferentation in the form of ganglionectomy would, theoretically, remove this focus of irritability.
Damage to a peripheral nerve causes changes central to the lesion that may not be reversed by treating the original injury.10 As noted earlier,25,35 these central changes include heightened sensitivity of the dorsal root ganglion to mechanical distortion and to neurotransmitters. Axons central to a nerve lesion also diminish their conduction velocity. Cells in the dorsal root ganglion may degenerate, with consequent degeneration of central axons. This leads to substantial loss of afferent fibers and produces deafferentation, which is another mechanism of pain. Additionally, the central terminals of C fibers change in response to peripheral nerve injury. The result is a failure of feedback mechanisms that produce prolonged depolarization and inhibition. Peripheral nerve section is thus followed by a reduction in inhibition of afferent fibers.37 The cord “responds” to diminished input (deafferentation) by diminishing inhibitions to the remaining input.38 The spinal cord itself thus becomes a location for continuing provocation of pain through mechanisms of chronic afferent barrage accompanied by reduced inhibition. Not surprisingly, many central ablative procedures such as cordotomy have been proposed as treatments for chronic pain syndromes. However, the role of these more central procedures in the treatment of chronic spinal pain syndromes is limited.
Finally, the individual motion segment is richly innervated and thus capable of generating postinjury pain in the absence of frank neural compression.39–44 Many of the procedures discussed next are intended for the treatment of continued extremity pain caused by persistent neurogenic dysfunction; such procedures are not, in general, successful in dealing with disorders of the motion segment per se. Thus entities such as posttraumatic lumbar strain, postdecompressive segmental instability, and persistent discogenic pain are not well served by deafferentation procedures. Additionally, reversible sources of neural compression producing continued sciatica such as disc herniation, lateral recess, or central or foraminal stenosis must be meticulously excluded before the consideration of any of these procedures. Indeed, the most effective way to deal with the “failed back” (failed back surgery syndrome, FBSS) is to avoid creating it by judicious and appropriately indicated initial treatment and surgery. Given the historical and current rates of spine surgery,45 in the settings of favorable natural history of many degenerative syndromes,46 it is unlikely that the incidence of FBSS will decrease.
Modulatory therapies that are germane to the concept of inhibition are nerve and cord stimulation and epidural implants. Destructive therapies are essentially deafferentation procedures: rhizotomy and ganglionectomy. More central ablative procedures have no place in the current treatment of failed lumbar surgery syndromes. For example, the dorsal root entry zone (DREZ) lesion, produced by electrocoagulation, has been reported to yield a success rate of 54% to 82% in brachial plexus avulsion47–50 and 50% in neurogenic pain from spinal cord injury; however, in benign pain syndromes or arachnoiditis, dismal results have been reported.51–54 Cordotomy55 has been extensively studied in cases of neoplastic pain and can be of major benefit in this instance.3,5,7,9 The procedure, in which the anterolateral pathways of the spinal cord are divided, thus interrupting pain and temperature transmission, has also been investigated for cases of lower cord or cauda equina injury. Porter and colleagues8 reported a 62% rate of significant pain relief in follow-up ranging from 8 to 20 years. White and Sweet cited a 60% rate of pain relief in patients with cord injuries and in four of seven with cauda equina damage. The complications of this procedure are significant, however: urinary incontinence, sexual dysfunction, and leg weakness. Additionally, genitourinary dysfunction rates of 8% to 92% have been reported.5,6,8,9
Deafferentation Procedures
Little attention in the recent literature has been focused on the use of rhizotomy as a treatment for chronic backache and sciatica. It has, however, been widely investigated in other areas. As noted previously, results in tumor patients are generally superior to those achieved in chronic benign pain patients.12,56–60 In a comprehensive review, Barrash and Leavens61 analyzed dorsal rhizotomy for relief of tumor pain. Promising results of rhizotomy were noted in cases of central neoplasms, as well as neoplasms involving the breast, colon, head and neck, lung, and rectal and urogenital systems. The problem of trigeminal neuralgia has been widely addressed as well. Van Loveren and colleagues62 reviewed their experience of 1000 patients with trigeminal neuralgia, comparing the techniques of percutaneous stereotactic rhizotomy and posterior fossa exploration. Of the 700 treated by percutaneous stereotactic rhizotomy, excellent or good results regarding pain relief were achieved in 125 patients treated with microsurgical vascular decompression or partial sensory rhizotomy. These favorable results were corroborated by the report of Bederson and Wilson63 in 252 patients. Additionally, glycerol rhizotomy for trigeminal neuralgia has been investigated.64–66 In general, good or excellent results are reported in 70% to 72% of the cases using this technique. Selective dorsal rhizotomy for spasticity in children with cerebral palsy, although controversial, has also been recommended. Cahan and colleagues67 and Kundi and colleagues68 emphasized the safety of the procedure, citing preservation of cortical somatosensory evoked responses. Good results have also been reported by others.11,17,69–71 Intraspinal rhizotomy has been reported to diminish spasticity in patients with myelomeningocele.72 Sacral rhizotomy in the treatment of hypertonic neurogenic bladder has also been investigated,31,73–75 as has control of spasticity resulting from posttraumatic paraplegia.76 Percutaneous radiofrequency rhizotomy resulted in improvement of spasticity in 24 of 25 patients in the series of Kadson and Lathi.77 In Turnbull’s series, percutaneous rhizotomy improved lower extremity spasm in paraparetic patients who were not hospitalized.78
Rhizotomy and Ganglionectomy
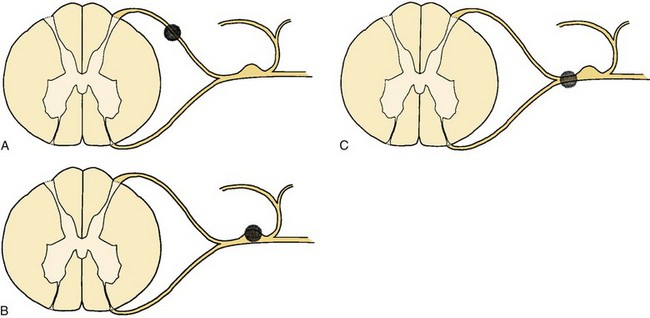
Sectioning of the spinal nerves or excision of dorsal root ganglia can be accomplished at multiple levels. Rhizotomy may be performed by intradural section of the dorsal root, extradural section of the dorsal root, or extradural section of the mixed root (Fig. 108–1). Additionally, the median branch of the posterior primary ramus may be interrupted, although this is usually by a percutaneous technique such as radiofrequency ablation (Fig. 108–2).
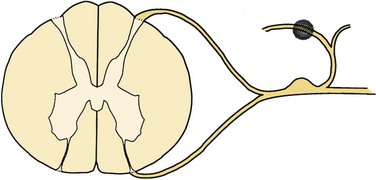
FIGURE 108–2 Facet rhizotomy. The shaded area represents the surgical lesion created.
(From Wetzel FT: Chronic benign cervical pain syndromes: Surgical considerations. Spine 17:S367-S374, 1992.)
Sensory rhizotomy for the relief of chronic pain was first carried out by Abbe in 1888 but had been nearly abandoned by 1925 because of the relatively high failure rate and the subsequent interest in cordotomy.11–79 Rhizotomy may be performed at this level to include selective sensory fibers, or it may take the form of a complete rhizotomy. Characteristically, both ablative procedures are performed proximal to the ganglion.14
The goal of rhizotomy or ganglionectomy is denervation of the area in which pain is felt. It has frequently been assumed that root section should remove pain that is peripheral and circumscribed because the afferent territory of a few adjacent nerves presumably completely delineates the pain for that region.14,80 Long-term results of rhizotomy fail to support this, however. In addition, results of selective sensory rhizotomy may be compromised because of the presence of denervation hypersensitivity, intersegmental cross-linking,16 and overlapping dermatomes81–84 and the presence of afferent unmyelinated axons in the ventral roots.85,86 Intraoperative stimulation of these roots has been shown to provoke pain. If these ventral afferents comprise a significant portion of the ventral root, dorsal sensory rhizotomy may be providing insufficient deafferentation to interrupt pertinent sensory pathways.
One of the central problems in planning surgery for persistent limb pain is the precise delineation of the involved roots. Many authors have attempted to select patients on the basis of their response to individual nerve root sheath blockade, as guided by electrophysiologic evidence of chronic radiculopathy and neurologic examination. Onofrio and Campa16 reported their results in 286 patients who underwent rhizotomy. Fifty-eight patients underwent lumbar rhizotomies. Only 6 of the 45 undergoing S1 rhizotomies were believed to have long-term pain relief. Three of 13 patients who underwent lumbar rhizotomies had clinically successful results. These results were obtained despite consideration of dermatomal overlap and the use of selective nerve root blockade to plan the surgery. Loeser15 reported similar results with a 14% success rate at 10 years. Arachnoiditis, in the setting of failed disc surgery, seemed to be correlated with poor results, and preoperative nerve root blocks provided little diagnostic or prognostic information. Loeser offered several reasons for these results including incomplete root sectioning, inadequate numbers of roots divided, and a higher threshold of fibers in adjacent nerves, which may begin to produce chronic pain syndromes after the effects of local anesthetics from root blocks have worn off. He also speculated that central alterations may be important. Additionally, the utility of “diagnostic” nerve root blocks must be questioned. The selective root sheath injection appears to be nonspecific in not only a dermatomal sense but in a central and peripheral sense as well: Several authors have reported on the ability of distal blocks to produce temporary relief.87–89 Jain90 believed that selective extradural sensory rhizotomy was not successful in the setting of arachnoiditis. Other authors have reported similarly discouraging results.13,88,91
In a compendium of results from multiple sources,11 Dubuisson noted a 74% rate of immediate success following rhizotomy at L4, L5, and S1, which dropped to 33% 3 months after surgery.14–16,59,90,91 These results are corroborated by the reports of others.13,88,91 White, in reviewing a series of sensory rhizotomies for 10 patients with failed lumbar surgery, noted 80% good to excellent results; however, follow-up was variable, ranging from 4 months to 11 years, and no temporal specifics were reported. He did, however, agree that there was little pain relief when arachnoiditis was present.57 Wetzel and colleagues92 reported poor outcomes (14% success) in patients undergoing selective sensory rhizotomy at a mean follow-up of 2 years. All patients had undergone previous unsuccessful lumbar surgery.
Thus it is difficult to recommend rhizotomy for the treatment of chronic benign lower extremity pain. Seemingly, the most reliable indication for rhizotomy is pain caused by deafferentation itself. Tasker and colleagues31 reviewed 168 patients. The pain was divided into two groups: spontaneous and hyperpathic. The latter implies pain production induced by normally non-noxious stimulation within adjacent areas of increased somatosensory thresholds. Overall, the pain in this group was nearly always causalgic or dysesthetic in quality and was associated with sensory loss. This was dramatically ameliorated by intravenous sodium thiopental, but not by morphine, and was usually relieved by proximal local anesthetic blockade. Various deafferentation procedures including rhizotomy, neurectomy, cordectomy, and cordotomy were reviewed, and each of these ablative procedures failed to relieve most patients of deafferentation pain. Hyperpathia, which occurred in incompletely deafferented areas, however, was partially relieved by surgical completion of the deafferentation, although the authors noted that pain may persist at the periphery of the sensory loss.
Sectioning of the dorsal root ganglion has been shown to provide the best results in terms of pain relief when performed for benign truncal neuralgias.93 The results of ganglionectomy (see Fig. 108–1) at the caudal lumbar roots in cases of failed lumbar surgery are as disappointing as those reported for rhizotomy.15,16,90,93,94 At this time, meaningful differentiation between rhizotomy and ganglionectomy as distinct therapeutic tools in this setting is impossible.
Technique
The patient is placed in a prone position under general anesthesia, and hemilaminectomy and partial facetectomy are used to expose the involved root. The root sheath is clearly identified and opened longitudinally for 8 to 10 mm proximal to the dorsal root ganglia. The dural septum, which separates dorsal and ventral roots, is identified, and a small nerve hook is passed between root filaments. Osgood and colleagues93 noted that several distinct root fascicles are usually present. With electrocautery at a low setting, electrical stimulation is used to distinguish between motor and sensory fibers. As Bertrand has noted, however, caution must be used in relying on this test exclusively because chronically damaged roots may exhibit a higher threshold for motor excitation response than normal roots.91 Thus a wake-up test may be required. When appropriate sensory fibers are identified, they can be sectioned with electrocautery or a microsurgical blade.
Facet Rhizotomy
Facet denervation is not nerve root surgery in the same sense that open rhizotomy is; rather, the theory behind this procedure involves destruction of the median branch of the posterior primary afferent nerve that supplies the facet joint (see Fig. 108–2). The median branch of the posterior primary ramus descends through a notch at the base of the transverse process and is covered by a ligament at the anteroinferior border of the facet joint at this level. This ligament is a continuation of the intertransverse membrane, and it is here that several small twigs are given off to the facet joint. These twigs then enter the facet joint capsule.44 Each posterior primary ramus supplies at least two facet joints, and each facet joint receives innervation from at least two spinal levels. Clinical features of facet joint syndrome have been described by several investigators. Mooney and Roberson are generally credited with one of the earliest descriptions.42 Subsequent authors have attempted to improve the sensitivity and specificity of diagnosis and investigate diagnostic maneuvers, specifically response to facet blockade as selection criteria for facet rhizotomy95 or even fusion.96 Saal, in reviewing current diagnostic techniques, noted that the capacity of diagnostic facet blocks to correlate specifically with findings of the history and physical examination is limited.97 Although a variety of signs and symptoms have been described as being associated with facet mediated pain, these signs or symptoms are not specific enough to delineate a patient population suffering from facet mediated pain per se.98,99 Several studies have noted that the prevalence of facet pain among patients with chronic back pain is relatively low when measured by facet injection; however, the anesthetic response to a single uncontrolled block is as high as 50%, an unacceptably high false-positive rate.100 It is generally felt that the “gold standard” by which facet joint blockade is judged is the appropriateness of the pain relief response based on duration of effect appropriate to the agent used for blockade and sustained relief by subsequent facet rhizotomy. This has been demonstrated in the cervical spine. In a study by Lord and colleagues,101 patients experiencing neck pain from whiplash, who responded to facet blocks, were randomized into active and sham groups for radiofrequency lesioning. The median duration of pain relief in the active group was significantly greater that in the sham group. No patients who failed to respond to blockade were included in the study.
Rees is generally credited with performing the first facet rhizotomies. These were done percutaneously with a knife and reportedly resulted in immediate relief of symptoms in 998 of the 1000 patients who had facet pain in concert with the “intervertebral disc syndrome.” Shealy102 performed the procedure in North America but had an unusually high frequency of wound hematomas. This led to the adoption of a radiofrequency probe. Success rates as high as 90% were initially reported in previously nonoperated patients. That pain relief was achieved by the interruption of afferent impulses of facet joint has been suggested by the anecdotal reports of many authors who have noted immediate relief of pain in patients undergoing lumbar fusion.
Candidates for facet rhizotomy are those patients with back pain caused by facet dysfunction who have failed to respond to conservative therapy.103 The key diagnostic maneuver is thought to be the facet block. This involves percutaneous insertion of a needle into the joint, under fluoroscopic guidance, followed by joint injection with lidocaine (Xylocaine) combined with steroids or contrast agents.42,104,105 Patients in whom this procedure yields temporary relief may be candidates for facet rhizotomy.103,104
In the series of Shealy,106 a satisfactory clinical result was noted in 79% of patients who had not had previous surgery. In patients who had undergone previous laminectomy, the success rate fell to 41%, and in those who had undergone previous fusion, success was only 27%. Of the 82 patients McCulloch followed from 6 to 20 months after facet rhizotomy, only 50% had satisfactory results.107 Interestingly, three patients in this group required repeat surgery. Schaerer reported on 71 patients who underwent lumbar facet rhizotomy.108 There were five distinct subgroups in his review: (1) lumbar facet disease without disc involvement (discography negative), (2) lumbar facet involvement with disc involvement (discography positive), (3) lumbar facet disease with discopathy and root signs, (4) facet signs with osteoarthritis, and (5) postlaminectomy pain. At a mean follow-up of 13.7 months, patients were evaluated using a pain profile. Thirty-five of 71 patients had satisfactory results. The highest percentage of success was in the author’s first group—those who had a “pure” facet syndrome (7 of 15 patients). No attempt was made to determine statistically significant differences in outcome between these groups. Florez and colleagues109 reported a series of 30 patients, achieving satisfactory results in 76%. Twenty-six of the patients were followed for 3 to 9 months. The best results were noted in patients without previous operations and those with shorter duration of symptoms. Oudenhoven110 reported 377 patients with “pseudoradicular” pain in whom a lumbar facet syndrome was diagnosed by facet blocks. At a mean follow-up of 26 months, 83% were judged to be clinical successes. The author noted that a unilateral facet rhizotomy did not control pain and reported that 22% of patients who were judged to be clinical successes noted some return of symptoms at 18 to 24 months postoperatively. None of the authors reported any significant complications with the procedure. Lord and colleagues111 reported on 19 patients who underwent cervical percutaneous neurotomy for neck pain of at least 3 months’ duration following motor vehicle accidents. They found that results varied by level. Of the 10 patients who underwent C2-C3 rhizotomy, 3 obtained greater than 6 months of pain relief and 1 was pain free at the 4-month follow-up; the remaining 6 had return of symptoms over 3 weeks. Of the 10 who underwent more caudal neurotomies, seven obtained “clinically useful” pain relief. The authors noted that C2-C3 results may have been compromised by technical failure including the relatively large diameter of the nerves and their variable course.
One may be tempted to recommend this procedure after diagnosis of facet syndrome with facet arthrography and blockade. The current literature, however, does not substantiate the rates of clinical success (83%) reported earlier.110 North and colleagues95 retrospectively reviewed prognostic factors for facet rhizotomy and found that only 42% of those selected for rhizotomy by response to facet blockade had pain relief at 2 years post procedure. Seventeen percent of those who experienced relief from blockade, but did not undergo rhizotomy, were improved. As noted previously, one randomized prospective study of rhizotomy for cervical pain noted a longer duration of relief in the active lesion group.101 The reported complication rates are low,112 and the apparent risk to the patient is minimal. There remain no convincing studies in the literature, however, suggesting that pain relief from the procedure is permanent.
Technique
The technique for this procedure is well described.103,107 It is recommended that it be performed in the operating room or radiology department. Local anesthetic is adequate; the patient should be in the supine position for cervical rhizotomy or the prone position for thoracic and lumbar rhizotomy. Image intensification is required. Fourteen-gauge needles are placed unilaterally in the region of the appropriate facet(s) and nerves. A 5-mm bare-tipped probe is then positioned in the area of the facet and the 14-gauge needle is partially withdrawn, leaving only the probe in the space between the superior facet and the transverse process immediately adjacent to the superior facet. The depth of the probe is controlled by lateral image intensification. A stimulation frequency of 100 Hz and from 0.1 to approximately 3 V is used to localize the tip away from the anterior ramus, as noted by the absence of paresthesia in the ipsilateral extremity. Once the depth is appropriate, stimulation adjacent to the posterior primary ramus reproduces a pain pattern familiar to the patient and the lesioning is then performed. A temperature-controlled lesion is produced by setting the controls at 25 V and 100 mA for approximately 60 seconds, at 80°C. During the final 20 seconds, the amperage is slowly increased to the point where the milliamperage starts to diminish and voltage rises. This takes the temperature to approximately 90°C. After this, the probe is withdrawn, the wound dressed, and the patient mobilized.
Sympathectomy
Sympathetic dystrophy represents a constellation of disorders of sympathetic nerve functions that accentuate or perpetuate chronic pain. Historically, Lankford113 divided sympathetic dystrophy into two types, causalgia and dystrophy, based on the type of injury. More recently, Stanton-Hicks and colleagues114 presented a revised taxonomic classification for reflex sympathetic dystrophy (RSD), regrouping the subtypes into a single entity, complex regional pain syndrome (CRPS). The diagnosis of CRPS requires regional pain and sensory changes following the index injury, coupled with skin color changes, temperature changes, abnormal sudomotor response, and edema. CRPS Type I occurs without a specific nervous lesion and corresponds to sympathetic or traumatic dystrophy; Type II refers to a discrete nervous lesion, equivalent to the former definition of causalgia. Obviously, many features of chronic spinal pain syndrome may fall into these various categories.
The anatomic constancy of the sympathetic trunk, with respect to the outer annulus, has been well demonstrated by Bogduk and colleagues.41 Pain fibers traveling lateral to the vertebral column in the sympathetic trunk may be prone to irritation owing to injury to the motion segment. Likewise, tears of the annulus fibrosus have been thought to be capable of producing a cold, painful limb on the ipsilateral side,39 and Hodgson described a pattern of intractable lower extremity pain, associated with diminished temperature, in patients with failed lumbar surgery.115
Sympathectomy has been investigated in the treatment of limb distress of other causes, most notably vascular disease. Norman and House reported the results of lumbar sympathectomy for peripheral vascular disease in 153 patients.116 Five years postoperatively, 67% of those who experienced claudication and 54% of those who experienced rest pain had avoided further surgery. Repelaer van Driel and colleagues117 reported favorable results from sympathectomy in 66 patients who had suffered from lower limb ischemia. Jones also noted a beneficial effect of digital sympathectomy in treating ischemia of the hand in systemic disease.118
In the setting of persistent neurogenic dysfunction, however, the results of sympathectomy have been far less predictable. A central problem remains in establishing the diagnosis. The sine qua non for diagnosis remains response to sympathetic blockade.40,87,119,120 Thermography has been suggested to be another diagnostic technique for detecting skin surface temperature changes associated with autonomic dysfunction.88,121,122 Even with the use of both modalities, however, doubts have been raised regarding the validity of patient selection in chronic lumbar pain syndromes.88
Mockus and colleagues123 reported on 34 patients who underwent lumbar sympathectomy. In 13, the precipitating incident was lumbar disc surgery. In this series, only one patient failed to obtain satisfactory relief. Wetzel and colleagues reported acceptable pain relief in only 4 of 17 patients at the 2-year follow-up. In this group, the patients’ response to block was not as significant in predicting response to surgery as was their initial thermographic diagnosis.88 Overall, including the results of even upper thoracic ganglionectomy, less than two thirds of patients report satisfactory pain relief at 2 years with less than one third reporting satisfactory pain relief at 5 years.112 Thus at the present time, lumbar sympathectomy cannot be recommended with any conviction in the setting of chronic benign lumbar pain syndromes.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree