Implant (year of FDA approval)
Material
Bearing surface
Articulations
Constraint
COR
Fixation
Cervical
ProDisc-C (2007)
CoCrMo
Metal on polymer
1
Semiconstrained
Fixed
Midline keel bone ingrowth
UHMWPE
Prestige (2007)
Stainless steel
Metal on metal
1
Semiconstrained
Mobile
Anterior screws
Bryan (2009)
Titanium
Metal on polymer
2
Unconstrained
Mobile
Milled cavities bone ingrowth
Polyurethane
Lumbar
SB Charité III (2004)
CoCrMo
Metal on polymer
2
Unconstrained
Mobile
Small fins/teeth bone ingrowth
UHMWPE
ProDisc-L (2006)
CoCrMo
Metal on polymer
1
Semiconstrained
Fixed
Midline keel bone ingrowth
UHMWPE
14.4.3 Disc Arthroplasty: Material Considerations and Bearing Surfaces
The development of a successful total disc arthroplasty device with acceptable long-term survival has relied on the immense clinical and technical experience of surgeons and engineers. The lessons learned from this field of study have helped to better understand and improve motion-preserving devices in the spine while avoiding some of the intrinsic pitfalls and complications especially in relation to the properties of materials and the mechanics of the system (Santos et al. 2004). The material properties necessary for any prosthesis must be determined based on the requirements expected of the implant (Taksali et al. 2004). For example, the lumbar spine sustains significantly greater loads than the cervical spine, whereas the cervical spine has a different kinematic pattern and greater range of motion. From this perspective, materials that perform well in the cervical spine may perform poorly in the lumbar spine.
Modern disc arthroplasty devices have a prosthesis-bone interface consisting of a broad rigid end plate made of metal; most devices are flat or slightly convex (dome) shaped. Not surprisingly, implant geometry is an important factor in disc arthroplasty, the goal being to maximize prosthesis-bone contact for bony ingrowths and to prevent subsidence. The primary alloys used in disc arthroplasty devices are stainless steel, cobalt chrome and titanium alloys. Cobalt chrome alloys have the greatest wear resistance, whereas titanium alloys have poor wear characteristics and surface hardness, making them unacceptable as an articulating surface. Titanium alloys demonstrate excellent biocompatibility with less susceptibility to bacterial surface colonization. Also, they generate a significantly less artifact during computed tomography or magnetic resonance imaging (Arens et al. 1996; Hallab et al. 2003a; Santos et al. 2004). They are most commonly used as a coating of the end-plate interface to facilitate bony ingrowth (Santos et al. 2004).
In terms of a bearing surface, the basic requirements is that the articular surface must allow for mobility, load distribution, low friction, and high wear resistance, as well as being biologically compatible with adequate longevity (Taksali et al. 2004; Lin and Wang 2006). Like total joint arthroplasty, the principal bearing surfaces of disc arthroplasty devices are metal-on-polymer or metal-on-metal articulations. Although ceramic-bearing surfaces have gained some popularity due to excellent wear characteristic, the brittle material properties and concerns for catastrophic failure in proximity to neural elements have precluded significant development (Garino 2000; Santos et al. 2004). In fact, a recent case report has demonstrated catastrophic failure of an experimental ceramic-bearing surface disc device implanted in a human patient (Nguyen et al. 2011).
The two primary polymers used for arthroplasty devices have been polyethylene and polyurethane. Ultrahigh molecular weight polyethylene has a good track record for several decades in extremity arthroplasty and has avoided complications associated with poor wear properties due to polyethylene sterilization techniques (Kurtz et al. 1999a, b; Hallab et al. 2003a; Taksali et al. 2004). Polyurethane has been used for decades for cardiovascular devices but has had limited use in spine surgery. Recently, the importance of its shock-absorbing properties has been recognized and incorporated into a disc arthroplasty device, in particular the Bryan cervical disc replacement (Medtronic Sofamor Danek, Memphis, TN). However, there are few studies evaluating axial motion and load transfer properties. This is unfortunate because one of the functions of the normal, healthy intervertebral disc is to provide shock absorption, and if absent may produce abnormal stress concentrations on surrounding structures within the segment and at the adjacent segment (Dahl et al. 2006). Dahl evaluated axial stiffness, energy absorption, and viscous damping of metal-on-polyurethane cervical disc arthroplasty with both a fusion construct and an intact intervertebral disc. The investigators found the dynamic stiffness of metal-on-polyurethane was similar to that of the intact disc, and energy absorption and viscous damping exceeded both the intact disc and fusion construct. In another study, Dahl et al. (2011) compared the shock-absorbing properties of polyurethane, polyethylene, and titanium alloy bearing surface materials. These workers demonstrated that polyurethane provides significantly lower stiffness and greater energy absorption and damping characteristics than polyethylene and titanium alloy. Of note, titanium alloy is currently not available as a bearing surface due to its poor wear characteristics. A concern with the use of polyurethane is the biologic durability or wear resistance. However, polyurethane has compared favorably to polyethylene and has been found to have excellent wear properties, with wear particles that do not incite a significant inflammatory response (Anderson et al. 2003, 2004; Taksali et al. 2004; Pitzen et al. 2007). While these reports acknowledged that shock absorption remains a theoretical benefit of disc arthroplasty devices, no clinical study has compared outcomes of shock- versus non-shock-absorbing devices (LeHuec et al. 2003).
Also of importance is the polymer core design, which predominately exists as either a single-gliding surface with a securely fixed polymer core or one with a metal end plate (ProDisc; Synthes Spine, Paoli, PA) or a double-gliding surface with a mobile polymer core sandwiched between two metal end plates (Bryan Cervical Disc; Medtronic Sofamor Danek, Memphis, TN). Concerns with the fixed polymer core are the presence of another point of implant failure due to constant micromotion, stress concentration, and a large difference in elastic properties at the metal-polymer interface. Problems associated with a mobile polymer core include concerns for polymer extrusion as well as increased debris from wear of the two articulating surfaces. Not surprisingly, there are continued research efforts to maximize the design properties and wear characteristics of metal-on-polymer bearing surfaces. Questions remain regarding wear debris and subsequent osteolysis with late implant failure, although the amount of debris from disc arthroplasty devices may be insignificant when compared to hip and knee joints due to the low range of motion and cyclic rate (Santos et al. 2004).
Despite concerns for systemic metal deposition (Wagner and Wagner 2000; Bisseling et al. 2011), adverse soft tissue reactions (i.e., pseudotumor) (Williams et al. 2011), and higher rates of early failure for some total hip replacement implants, metal-on-metal articulations have been used in total hip arthroplasty devices (Bernthal et al. 2012; Langton et al. 2011). The advantage of a metal-on-metal articulation compared to metal-on-polymer bearing surface is the significantly lower (approximately 10 times) wear rate (Goldsmith et al. 2000; Santos et al. 2004; Taksali et al. 2004). However, there have been reports of elevated systemic metal ion levels in patients following lumbar metal-on-metal total disc replacement (Wagner and Wagner 2000; Bisseling et al. 2011). A recent review of complications in metal-on-metal disc arthroplasty devices reported abnormal inflammatory reactions and soft tissue masses with lymphocyte or macrophage infiltration, similar to those found in patients following metal-on-metal bearing surface total hip arthroplasty (Golish and Anderson 2012). Because of this paucity of information, the surgical community awaits information on the long-term outcomes and complication profile concerning the use of metal-on-metal disc arthroplasty devices.
14.4.4 Semiconstrained Versus Unconstrained Devices
Similar to total joint arthroplasty implants, the amount of constraint is an important factor in disc arthroplasty design. With increasing constraint in total joint arthroplasty, the trade-off is greater stability for greater stress on the implant-bone interface. For disc arthroplasty, there are both semiconstrained and unconstrained designs, with each having advantages and disadvantages (Huang et al. 2003; Santos et al. 2004). The unconstrained design provides a mobile instantaneous axis of rotation and is more representative of the physiologic axis, which was demonstrated by Gertzbein et al. (1986) to be an ellipse rather than a single point. Theoretically, an unconstrained device would provide a greater range of motion and may be more tolerant of small errors in implant placement (Hallab et al. 2003a; Huang et al. 2003). However, an unconstrained articulation subjects the facets and posterior ligaments to increased shear and torsional loads, whereas semiconstrained devices unload the facet joints and ligaments (Cunningham et al. 2003a; Huang et al. 2003; Polly 2003; Santos et al. 2004). Semiconstrained disc arthroplasty devices have increased stability, but similar to joint arthroplasty there is higher load transfer to the implant-bone interface. Currently, the ideal amount of constraint remains unknown and will eventually be determined by long-term clinical studies.
14.4.5 Implant Fixation
Fixation of the disc arthroplasty device to host bone is another important factor to consider, taking into account both initial and long-term implant stability (Santos et al. 2004). Unlike total joint arthroplasty which has successfully used cemented knee arthroplasty implants and cemented femoral stem implants as originally advocated by Charnley (Schulte et al. 1993), disc arthroplasty has not been performed with cement fixation because of the proximity to neural elements (Santos et al. 2004). Another reason cement fixation may be inappropriate for disc arthroplasty is because of the younger patient population compared to extremity arthroplasty, which may potentially increase the incidence of cement fatigue and aseptic loosening due to greater activity levels and physical demands (MacWilliam et al. 1996; McLaughlin and Lee 2000; Santos et al. 2004).
Therefore, immediate stability is obtained by screw fixation of metal end plates to the anterior vertebral body in some devices, whereas others have end-plate designs with a midline fin/keel, or spikes that project perpendicular to the end plate (Lee and Goel 2004). There have been some concerns regarding screw fixation, particularly in the lumbar spine which presents a greater risk for major vascular complications. In the cervical spine, due to the increased anterior profile, there is the possibility of causing dysphagia; due to fixation on the anterior cervical body, difficulties may be experienced in revision surgery in the setting of adjacent segment disease (Kulkarni et al. 2003; van Ooij et al. 2003; Bertagnoli et al. 2005b; Patel et al. 2008).
Long-term stability requires osseointegration achieved through porous ingrowth surfaces or on-growth surface coatings (Taksali et al. 2004). The basic requirements of successful bone ingrowth include implant stability, optimal pore size, and optimal surface geometry/surface area (Kienapfel et al. 1999; Santos et al. 2004). Surface coatings to improve bone on-growth include roughened titanium, titanium wire mesh, plasma-sprayed titanium, and bioactive materials such as hydroxyapatite and calcium phosphate (Taksali et al. 2004). Animal studies have shown successful bone integration with titanium- or hydroxyapatite-coated surfaces (Cunningham et al. 2002, 2003a; Santos et al. 2004). In fact, Cunningham et al. (2002) reported that a lumbar disc arthroplasty device with porous-coated titanium end plates in a nonhuman primate model evidenced bone ingrowth into 56 % of the end-plate surface at 12 months. This is comparable to total joint replacement components, which have found cementless femoral and acetabular implant ingrowth of 9.7–33 % (Sumner et al. 1990; Harvey et al. 1999) and 12 % (Pidhorz et al. 1993), respectively. However, these coatings should continue to be carefully evaluated to ensure acceptable tensile strength, shear strength, and fatigue strength. The potential concern is that inadequate surface coating integrity may cause migration of coating material debris in between the articular surface, leading to accelerated third-body wear (Taksali et al. 2004).
14.5 Comparison of Total Disc Arthroplasty Devices
Cervical and lumbar disc replacements have been categorized as “significant risk devices” by the United States Food and Drug Administration (FDA) and require both preclinical and clinical evaluations to determine their safety and effectiveness (Orr et al. 2007). As noted previously, many of these devices have been used in Europe for decades, and only recent interest in the United States has resulted in several ongoing FDA-sponsored clinical trials, comparing the outcomes and safety of disc arthroplasty devices, using fusion procedures as the control. Although there have been over 100 disc arthroplasty designs and patents, to date there have been two lumbar disc replacement devices (SB Charité III, DePuy Spine, Raynham, MA; and ProDisc-L, Synthes Spine, Paoli, PA) and three cervical disc replacement devices cleared for marketing (Prestige, Medtronic Sofamor Danek, Memphis, TN; ProDisc-C, Synthes Spine, Paoli, PA; Bryan, Medtronic Sofamor Danek, Memphis, TN) by the FDA (Orr et al. 2007). All devices have demonstrated that human use for one-level degenerative disc disease is “non-inferior” to the fusion control group (Orr et al. 2007). We will further discuss the specific design considerations for each FDA-approved device.
14.5.1 Box 14.1 FDA Investigational Device Exemption (IDE), Premarket Approval, and 510(k) Notification
Cervical and lumbar disc replacements are categorized as “significant risk devices” (Class III) by the US Food and Drug Administration (FDA) and have required both preclinical and clinical evaluations to determine their safety and effectiveness prior to marketing. The FDA authorizes an investigational device exemption (IDE), which allows a device to be used for the purpose of a clinical study to collect safety and effectiveness data to support a premarket approval or premarket notification 510(k). When under IDE status, the device remains unapproved by the FDA and typically can only be used on human subjects when it is under clinical investigation and used by investigators participating in the clinical trial. However, the FDA also allows the use of an unapproved device before premarket approval for “emergency use” to save the life of a patient or prevent irreversible morbidity, for “compassionate use” to help a patient suffering from a serious disease or condition for which there exists no other alternative therapy, and after an IDE clinical trial for “continued access” if there is a public health need, and preliminary evidence suggests the device will be effective with no significant safety concerns.
There have been only a few total disc arthroplasty devices which have received FDA premarket approval, which is a rigorous process of scientific and regulatory review to evaluate the safety and effectiveness of Class III medical devices. The completion of a premarket approval for a device usually requires the manufacturer to dedicate a significant amount of time, energy, and funding. However, the FDA allows certain modified devices to undergo the 510(k) notification process which relies on comparisons with an already-approved and similar device and provides an expedited review, usually within 90 days. The 510(k) notification does not require the modified device to undergo extensive clinical testing, thereby avoiding the rigorous premarket approval process. Many manufacturers attempt 510(k) notifications for their devices because of the ability to rapidly market their product; however, this process may permit a modified device to be used in a widespread fashion without a full understanding of the risks, failure rate, and complications.
14.5.2 Lumbar TDA Devices
The SB Charité III (DePuy Spine, Raynham, MA) (Fig. 14.1) was the first total disc arthroplasty device to be implanted in the USA in 2000 as part of a controlled randomized study and was subsequently the first FDA-approved disc arthroplasty device in 2004. SB Charité has evolved from its initial design (SB Charité I and II) designed by Shellnack and Büttner-Janz in the early 1980s, which consisted of small, shell-like end plates made of stainless steel, which resulted in implant subsidence and migration (Buttner-Janz et al. 2002; Link 2002). The Charité II attempted to address this problem by adding thin lateral wings to increase the surface area of the end plates; however, these wings developed early fatigue fractures (Lin and Wang 2006). The device is now in its third and current version and was developed in 1987 with broad flat end plates manufactured from cobalt-chromium-molybdenum (CoCrMo) alloy with small teeth projecting into the vertebral end plates (Lin and Wang 2006). There is also the new InMotion design, which retains the essential characteristics of the Charité III, with the modifications involving the teeth orientation and the addition of a central rail portion allowing the prosthesis to glide onto a ramp inserter (Serhan et al. 2011). The device end plates are porous-coated with plasma-sprayed titanium and calcium phosphate (TiCaP) to assist with bony ingrowth. The polyethylene core is a sliding, unconstrained biconvex design to allow for an instantaneous axis of rotation (IAR) that permits anterior and posterior movement to the midpoint of the disc during flexion and extension, respectively, which is believed to more closely replicate normal segmental motion (Lin and Wang 2006). However, the IAR of the arthroplasty device has been found to be more anterior than normal, in both flexion and extension, and may detract from its presumed advantage (Bono and Garfin 2004). Cunningham showed that the motion of the Charité III prosthesis closely resembles normal motion in cadaveric testing, with disc motion similar to the intact segment in flexion-extension and lateral bending; however, normal limits of axial rotation were exceeded (Cunningham 2004). Another disadvantage is that there are two articulating surfaces which theoretically may increase polyethylene wear and debris formation, compared to only one gliding surface. Also, the polyethylene core is unconstrained with the potentially catastrophic complication of core extrusion.
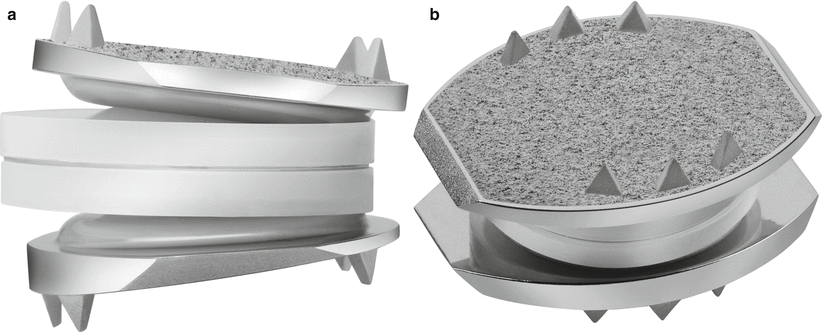

Fig. 14.1
(a, b) SB Charité III lumbar disc arthroplasty device (Images provided by DePuy Spine, Raynham, MA)
ProDisc-L (Synthes Spine, Paoli, PA) (Fig. 14.2) was designed and developed by Marnay in the 1980s and first implanted in France in 1990. The device received FDA approval in 2006 after undergoing several design modifications, including a change of end-plate material from titanium to CoCrMo and the addition of ultrahigh molecular weight polyethylene (UHMWPE) bearing surface as a separate modular piece which is snap-locked to the inferior end plate (Lin and Wang 2006). A single, titanium plasma spray-coated midline sagittal fin is used to improve immediate and long-term end-plate fixation, as opposed to the six small teeth of the SB Charité III. The semiconstrained interface between the polyethylene core fixed to the inferior end plate articulating with a polished superior metallic end plate is thought to decrease the risk of polymer extrusion. However, the rotational axis lies within the anterosuperior aspect of the lower vertebral body, and the fixed axis of rotation does not allow for an anatomic coupled anteroposterior translation with flexion and extension (Lin and Wang 2006). In patients with a degenerative spinal motion segment with abnormally increased range of motion, the semiconstrained design is thought to be beneficial by allowing stability through a more controlled arc of motion and protecting the facet joints from shear forces. However, some authorities believe that increased constraint may cause abnormal force transfer to the bone-end plate interface resulting in premature loosening, abnormal forces within the facet joints, and anteroposterior dimensional changes of the neuroforamina during motion (Huang et al. 2003; Bono and Garfin 2004).
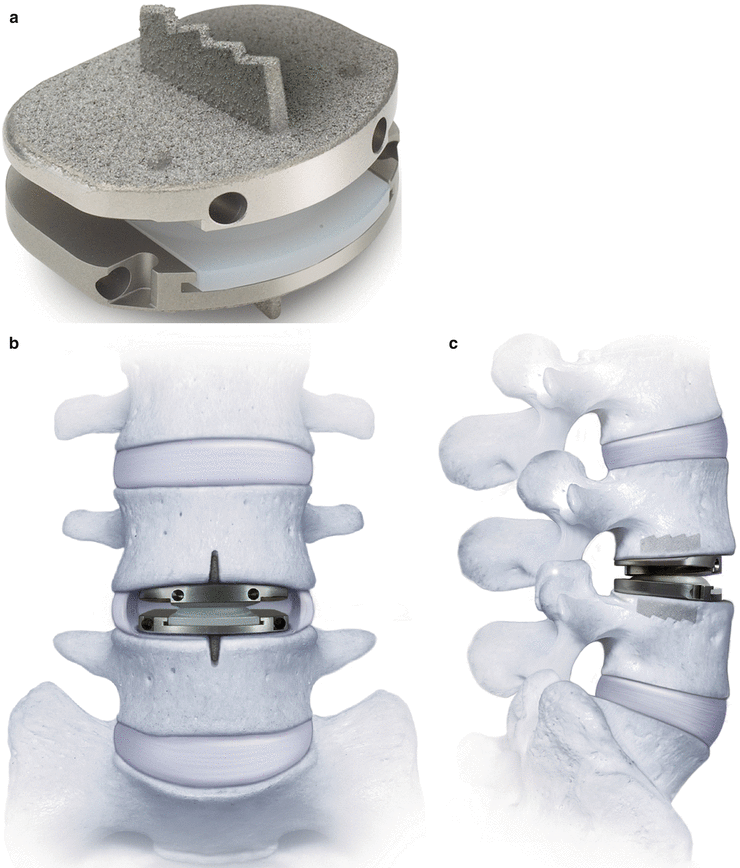

Fig. 14.2
(a–c) ProDisc-L lumbar disc arthroplasty device (Images provided by Synthes Spine, Paoli, PA)
14.5.3 Cervical TDA Devices
ProDisc-C (Synthes Spine, Paoli, PA) (Figs. 14.3 and 14.4) is similar in design to its lumbar counterpart and received FDA approval in 2007. The device is also made up of three components, which include an inferior and superior CoCrMo alloy end plate with a midline keel-oriented anteroposterior anchoring into the end plate of the respective vertebral body. In addition there is a highly polished concave bearing surface from the superior alloy end plate which articulates with a convex (spherical dome) UHMWPE insert that is preassembled and snap-locked into the inferior alloy end plate (Lin and Wang 2006). Similar concerns exist regarding the semiconstrained articulation and may be of greater concern in the cervical spine due to the inherent greater range of motion; however, this has yet to be evaluated.
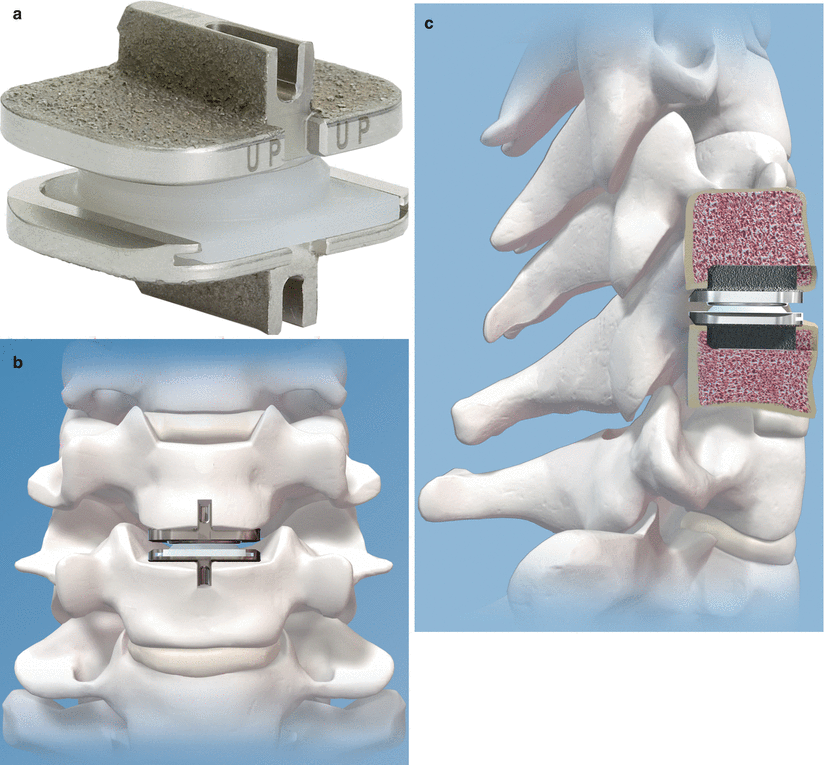
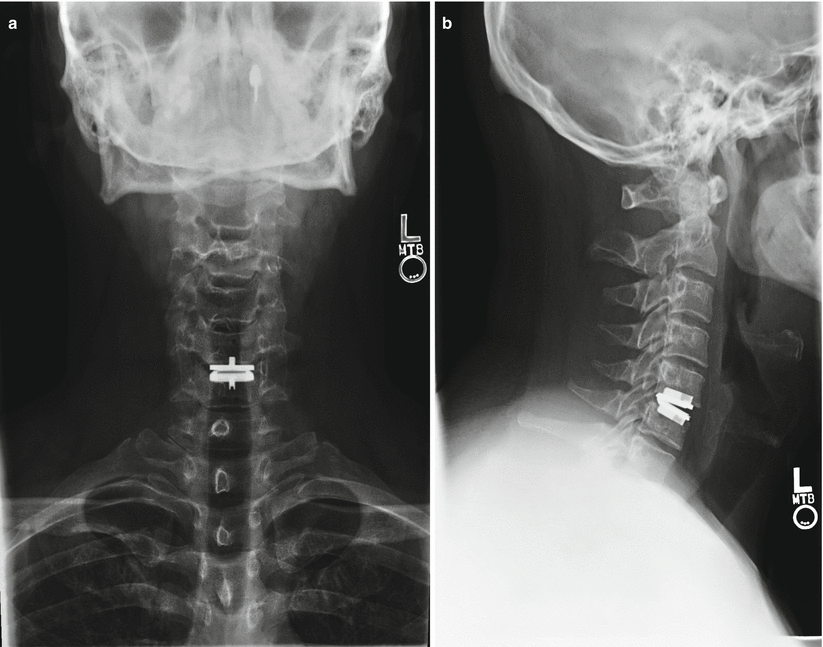

Fig. 14.3
(a-c) ProDisc-C cervical disc arthroplasty device (Images provided by Synthes Spine, Paoli, PA)

Fig. 14.4
(a, b) Radiographs of patient with single-level ProDisc-C device
The Prestige ST (Medtronic Sofamor Danek, Memphis, TN) (Figs. 14.5 and 14.6) was developed by Gill and colleagues in 2002 and was approved by the FDA in 2007. The Prestige ST is a modification of the original Prestige I (1989) and II (1999) initially designed and developed by Bristol-Cummins at Frenchay Hospital in 1989 (Traynelis 2004). The key improvements between Prestige I and Prestige II were a more anatomic end-plate design, which was roughened/grit-blasted to promote bony ingrowth; an improvement from Prestige II to Prestige ST was a 2-mm reduction in the height of each anterior flange. The device is a semiconstrained metal-on-metal prosthesis (stainless steels), with a ball-in-trough design which is postulated to replicate physiologic segmental motion (Traynelis 2004). To provide immediate stability, and unique to other designs, screws are placed through plate-like extensions on the superior and inferior, anterior vertebral bodies (Bono and Garfin 2004). The ball-in-trough design provides semiconstrained motion; however, unlike the ProDisc ball-in-socket design, there is some coupled translation with flexion-extension. Again, the consequences of this added translation are unknown, and further long-term follow-up is necessary to determine if there are detrimental effects on the facets joints from shear forces.

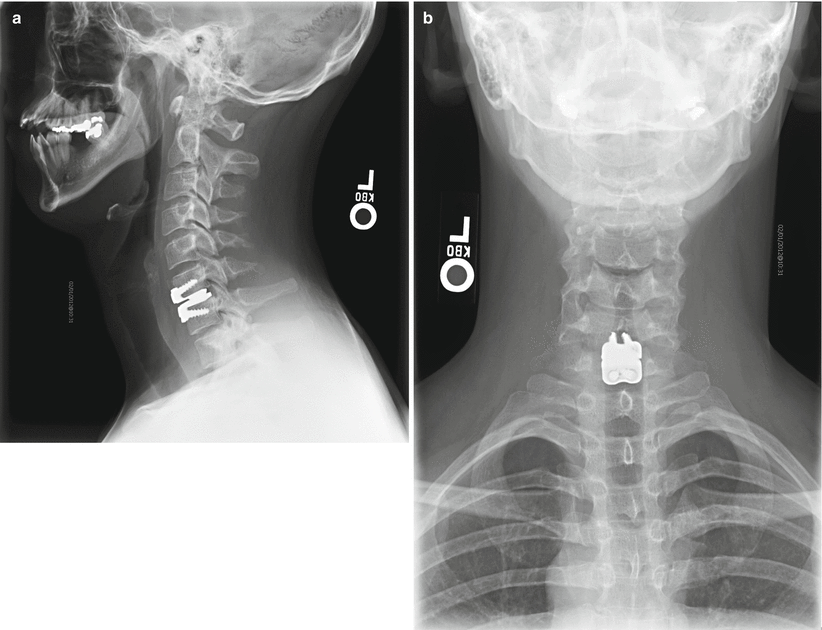

Fig. 14.5
Prestige ST cervical disc arthroplasty device (Images provided by Medtronic Sofamor Danek USA, Inc.)

Fig. 14.6
(a, b) Radiographs of patient with single-level Prestige ST cervical disc device
The Bryan Cervical Disc (Medtronic Sofamor Danek, Memphis, TN) (Figs. 14.7 and 14.8) was developed in the late 1990s and received FDA approval in 2009. The device is an unconstrained, biarticulating metal-on-polyurethane prosthesis. It is composed of two titanium alloy shells, a polycarbonate polyurethane nucleus, and a polyether polyurethane sheath with titanium-retaining wires. The polyurethane sheath spans between the metal end plates and surrounds the nucleus, forming a cavity that is filled with saline, acting as synovial fluid or lubricant (Bono and Garfin 2004). This is thought to keep potential wear debris within the cavity while also preventing soft tissue ingrowth between the articulating surfaces (Lin and Wang 2006). The device also has a unique method of fixation to the bone, sitting in a pocket milled into the vertebral end plate; long-term stability is provided by bone incorporation with a beaded titanium coating on the device end plates – it is not screwed or secured by teeth to the vertebra. As previously discussed, the polyurethane nucleus provides greater shock-absorption and load-dampening properties compared to polyethylene and metal bearing surfaces; however, the clinical benefits have not been established.

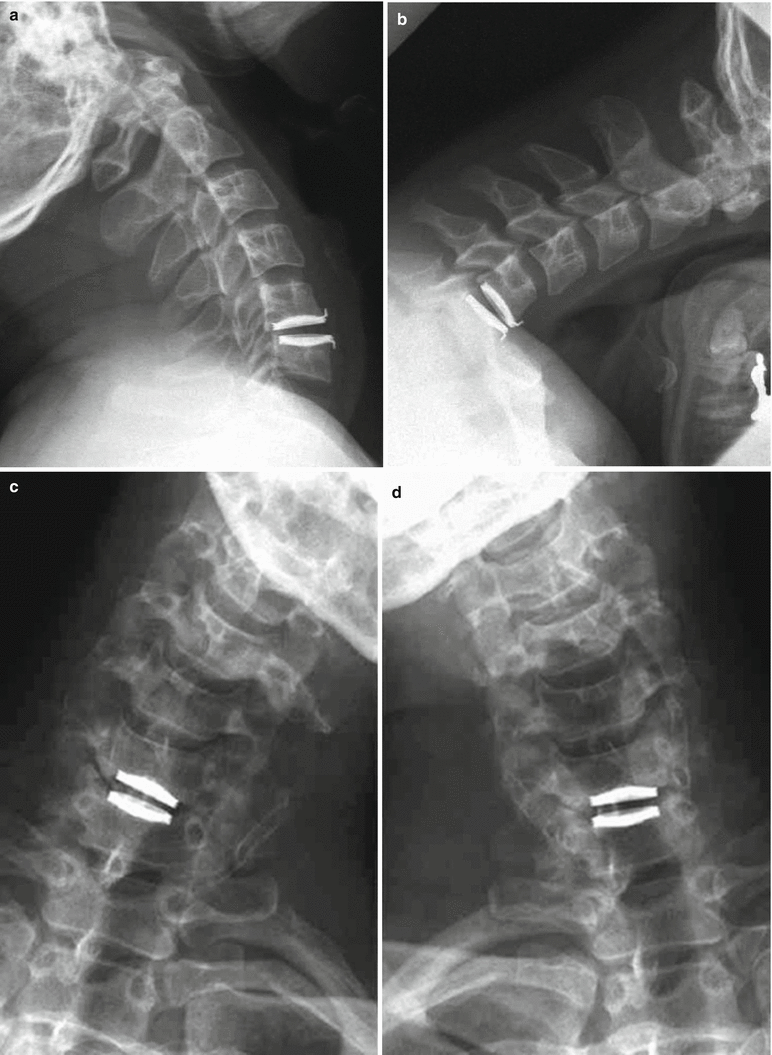

Fig. 14.7
Bryan cervical disc arthroplasty device (Images provided by Medtronic Sofamor Danek USA, Inc.)

Fig. 14.8
(a–d) Radiographs of patient with single-level Bryan cervical disc arthroplasty device (Images provided by Medtronic Sofamor Danek USA, Inc.)
14.5.3.1 Box 14.2 Spinal Device Registry
The development of a spinal device registry within the USA remains a necessity, particularly with the real and potential benefits demonstrated by registries for other medical devices. Registries for hip and knee replacement devices have become a worldwide reality with preeminent registries in Sweden, Finland, Norway, Australia, Denmark, and New Zealand, approaching 15 years of experience. A device registry allows a real-time assessment of the current clinical practice and associated outcomes, providing timely feedback, and permits healthcare organizations to significantly influence physician behavior. Most importantly, to avoid unnecessary complications for patients, device registries provide an early warning system for early implant failure. Device registries also validate the value and cost-effectiveness for the use of healthcare resources and promote improved clarity and evidence in the development of clinical practice guidelines.
The Swiss Spine Registry is the first national spine device registry started in March 2005 to gather data on patient outcomes, cost, and performance information for cervical and lumbar total disc arthroplasty and balloon kyphoplasty procedures. A 3-year pilot study with 135 participating Swiss surgeons demonstrated an 80 % data capture rate for total disc arthroplasty cases performed (925 cervical and 497 lumbar disc replacements), and analysis of registry data generated sufficient evidence to validate continued federal health insurance reimbursement. The success of the Swiss spine registry should serve as an example for the development of a US spinal device registry and collection of observational data in a nationwide framework to enhance the quality of patient care and tracking the performance of spinal implants.
14.5.3.2 Box 14.3 Evolution of Total Disc Arthroplasty Devices: Timeline
1950s Fernstrom ball, considered the first rudimentary intervertebral disc prosthesis
1978 Fassio develops disc arthroplasty device with a central silastic compressible ball
1997 Kostuik develops a device with an articulating hinge and springs between two metallic end plates; however, it was never implanted in humans
SB Charite Lumbar Disc Arthroplasty
Early 1980s SB Charité I lumbar disc arthroplasty device developed by Büttner-Janz and Schellnack
1987 SB Charité III, third generation and current version was developed
2000 SB Charité III, first total disc arthroplasty device implanted in the USA
2004 SB Charité III, approved by US FDA for marketing
2011 SB Charité III, removed from market by manufacturer
ProDisc-L and ProDisc-C Disc Arthroplasty
1980s ProDisc-L, developed by Marnay
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








