(1)
Division of Orthopaedic Surgery, Lady Davis Institute for Medical Research, McGill University, 3755 Chemin de la Cote Ste-Catherine, Montreal, QC, H3T 1E2, Canada
5.1 Introduction
5.2.3 Other Matrix Molecules
5.5.3 Fibril Formation
5.5.4 Cross-Link Formation
5.8.1 Hereditary Disorders
Abstract
Characterized by the presence of at least one triple-helical domain, members of the collagen family are the most abundant proteins in the animal kingdom. From a phylogenetic perspective, while the triple-helical domain is present in bacteria and fungi and even some viruses, collagen and collagen-like proteins have been identified in all metazoa. With the appearance of the phylum Chordata, the notochord provided a “first” skeleton which enabled the organism to assume a longitudinal shape and provided support for the digestive tube and the nerve cord. This notochordal structure is sheathed in collagen; postembryonic remnants of the notochord in vertebrates form the nucleus pulposus of the intervertebral discs. Zhang et al. (2009) have put forward the hypothesis that the vertebrate chondrocytes that all express the type II gene may have evolved from notochordal cells. In vertebrates, collagen fibrils formed the template for deposition of mineral and the development of bone and cartilage of the axial and appendicular skeleton.
5.1 Introduction
5.1.1 Evolutionary Considerations
Characterized by the presence of at least one triple-helical domain, members of the collagen family are the most abundant proteins in the animal kingdom. From a phylogenetic perspective, while the triple-helical domain is present in bacteria and fungi and even some viruses, collagen and collagen-like proteins have been identified in all metazoa. With the appearance of the phylum Chordata, the notochord provided a “first” skeleton which enabled the organism to assume a longitudinal shape and provided support for the digestive tube and the nerve cord. This notochordal structure is sheathed in collagen; postembryonic remnants of the notochord in vertebrates form the nucleus pulposus of the intervertebral discs. Zhang et al. (2009) have put forward the hypothesis that the vertebrate chondrocytes that all express the type II gene may have evolved from notochordal cells. In vertebrates, collagen fibrils formed the template for deposition of mineral and the development of bone and cartilage of the axial and appendicular skeleton.
5.1.1.1 Box 5.1 Abbreviation List
C-NC domain
Carboxyl-terminal noncollagenous domain (same as C-propeptide)
COMP
Cartilage oligomeric matrix protein
DDR
Discoidin domain receptors
ECM
Extracellular matrix
ED-A
Extra domain A
EGF
Epidermal growth factor
ER
Endoplasmic reticulum
FACIT
Fibril-associated collagen with interrupted triple helices
GAG
Glycosaminoglycan
IVDD
Intervertebral disc degeneration
MACIT
Membrane-associated collagen with interrupted triple helices
MMP
Matrix metalloproteinases
Multiplexin
Multiple triple-helix domains and interruptions
NC
Noncollagenous
N-NC domain
Amino-terminal noncollagenous domain (same as N-propeptide)
RER
Rough endoplasmic reticulum
RUNX2
Runt-related transcription factor 2
TIMP
Tissue inhibitors of metalloproteinases
5.1.2 Brief Overview of the Anatomical Characteristics of the Intervertebral Disc
The discs typically are composed of three morphologically distinct major structural regions – the peripheral collagen-rich annulus fibrosus, surrounding the proteoglycan-rich central nucleus pulposus, and the cartilage end plates interfacing the disc and the vertebral body. The junctional zone between vertebral bone and annulus fibrosus is a type of enthesis. The cartilage end plates in the juvenile are responsible for longitudinal vertebral growth. In the adult, the intervertebral discs are avascular and receive most of their nutrients from the vertebral bone vasculature by diffusion through the cartilage end plates. Collagens provide the structural framework of the intervertebral discs and are responsible for its biomechanical properties such as torsion and resistance to pressure or tension. Proteoglycans, such as decorin, fibromodulin, and biglycan together with other matrix constituents, have important influences on collagen fibril formation.
5.1.2.1 Annulus Fibrosus
The annulus fibrosus makes up the peripheral portion of the disc structure (Fig. 5.1). In the mature lumbar disc, there are up to 25 lamellae that comprise regular concentric bundles of parallel collagen fibers arranged around the central gelatinous nucleus pulposus (Roberts 2002). Collagen fibers in a particular lamella run in one direction, while fibers in an adjacent lamella run in the opposite direction. The thickness of the lamellae varies from 200 to 400 μm, increasing from inside to outside (Inoue 1973). This alternating pattern is designed to withstand torsional stresses. The key functions of the collagen fibrils in the annulus are retaining the nucleus and taking up and distributing the load exerted by the disc.
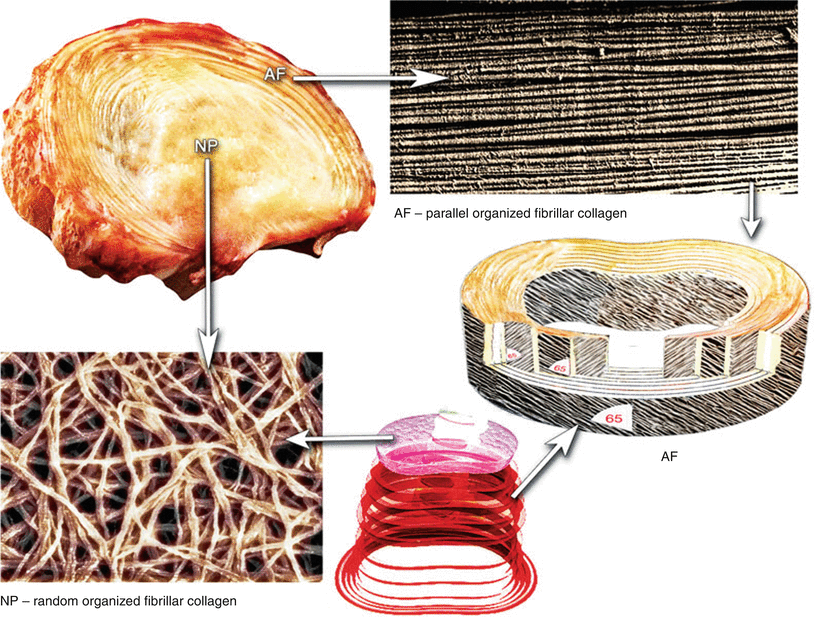

Fig. 5.1
Schematic illustration of the organization of fibrillar collagens in the annulus fibrosus (AF) and nucleus pulposus (NP) of a human intervertebral disc
5.1.2.2 Nucleus Pulposus
The nucleus pulposus is a soft jellylike, highly hydrophilic tissue occupying the central region of the disc. The disc contains proteoglycans (predominantly aggrecan; see Chap. 4), randomly organized fibrillar collagens (Inoue 1981), radially arranged elastin fibers (Yu et al. 2002), and water. The proportion and organization of water, fibrillar collagens, and proteoglycans vary not only with the position across the disc, but with age and level (Antoniou et al. 1996; Scott et al. 1994; Demers et al. 2004; Mwale et al. 2004; Antoniou et al. 1996). The nucleus pulposus has a higher concentration of proteoglycans and water than other regions of the disc, whereas the highest levels of collagen are in the outer annulus and the lowest concentration in the nucleus (Mwale et al. 2004; Inkinen et al. 1998). The collagen content of the nucleus pulposus is greatest in cervical discs and lowest in lumbar discs. In contrast, the proteoglycan content of the nucleus pulposus peaks in lumbar discs and is lowest in cervical discs (Scott et al. 1994).
5.1.2.3 Cartilage End Plates
The end plates in the human disc consist of a thin horizontal layer of hyaline cartilage, usually less than 1 mm thick, which separate the nucleus pulposus and annulus fibrosus from the adjacent vertebral bone. In the adult, the cartilage end plate is narrow, and often calcified, leading to disturbances to the nutrient supply to the nucleus pulposus (Maroudas et al. 1975; Nachemson et al. 1970). The collagen fibers within the end plates run parallel to the vertebral bodies, with the fibers continuing into the disc (Roberts et al. 1989).
5.2 Protein Composition of the Intervertebral Discs
5.2.1 Collagenous Protein Structure
Collagen, the most abundant fibrous protein of the disc, is the name for a family of rope-like polypeptidic molecules (Fig. 5.1) that have a linear structure containing regions of repeating triplets of amino acids (GlyaaXaaYaa) with glycine (Gaa) at every third position and X and Y often being proline and hydroxyproline, respectively. These two amino acids constitute about 1/6 with glycine accounting for 1/3 of the sequence. Since glycine is the smallest amino acid with no side chain, it is positioned at every third position in the chain. The high glycine content stabilizes the collagen helix, facilitating hydrogen bonding and the formation of intermolecular cross-links (Eyre et al. 1984; Pokharna and Phillips 1998; Berg and Prockop 1973). The ring structures of proline and hydroxyproline occupy the outer positions where they interact with neighboring collagen molecules. The hydroxyl groups of 4-hydroxyproline are essential for the formation of intramolecular hydrogen bonds and determine the thermal stability of each collagen chain (Berg and Prockop 1973).
The collagen molecule is formed from three α-chains wound together in a triple helix. These helices are twisted together into a right-handed coiled coil stabilized by hydrogen bonds and woven together to form a left-handed helix (Hulmes and Miller 1981). This uninterrupted triple-helix molecule is approximately 300 nm in length and 1.5 nm in diameter – a cable-like structure that provides significant tensile strength to skin, tendons, ligaments, and other cartilaginous structures, making them tough and flexible. The amino acid hydroxylysine is responsible for stabilizing the side-to-side packing of the chains into fibrils. The collagen molecule contains three structural domains, the amino- and carboxyl-terminal extra-helical regions and the major triple-helical rodlike domain. Collagens I–V are considered to be the major collagens, since they comprise 98 % of the total connective tissue protein. There is considerable complexity and diversity in the 28 different types of collagen.
In the lumbar disc, there are up to 25 lamellae that comprise regular concentric bundles of parallel collagen fibers arranged around the central gelatinous nucleus pulposus (Roberts 2002). Collagen fibers in a particular lamella run in one direction, while fibers in an adjacent lamella run in the opposite direction. The thickness of the lamellae varies from 200 to 400 μm, increasing from inside to outside (Inoue 1973). This alternating pattern is designed to withstand torsional stresses. The key functions of the collagen fibrils in the annulus are retaining the nucleus and taking up and distributing the load exerted by the disc. The fibril-forming collagens I and II form an important network in the disc. Collagen I forms hybrids with other fibrillar collagens, particularly collagen V. Co-fibril formation with collagen I is believed to regulate the diameter of fibrils because of the partial processing of the N-propeptide of collagen V. With disc aging and degeneration, there are profound alterations in collagen cross-links (Pokharna and Phillips 1998).
The proportion of collagen II and I varies gradually and inversely across the disc, with exclusively collagen I at the extreme outermost layers of the annulus fibrosus and the nucleus pulposus possessing mostly collagen type II (Eyre and Muir 1976). Under physiological conditions, collagen II fibrils contain more water than I fibrils (Grynpas et al. 1980). Collagen V is a minor component and forms hybrids with collagen I, while XI forms hybrids with collagen II.
The collagen chains are synthesized as procollagens. In the rough endoplasmic reticulum (ER), the procollagen chain undergoes a series of processing reactions. First, as with other secreted proteins, glycosylation of procollagen occurs in the rough ER and Golgi complex. Galactose and glucose residues are added to hydroxylysine residues, and long oligosaccharides are added to specific asparagine residues in the carboxyl-terminal propeptide, a segment at the carboxyl-terminus of a procollagen molecule that is absent from mature collagen. Generally, the propeptides (amino and carboxyl terminal) are removed after secretion, and then collagen fibrils form in the extracellular space.
5.2.2 Classification of Collagens
The disc contains many different collagen types whose abundance changes with age (Roughley 2004). The annulus fibrosus is composed primarily of collagen I and to a lesser extent II, III, V, VI, IX, XI, XII, and XIV. The nucleus pulposus is rich in collagen II, but it also contains collagen I, VI, and IX (Eyre and Muir 1976, 1977; Wu et al. 1987; Eyre et al. 2002). Collagens I and II constitute about 80 % of the collagens in the disc (Eyre and Muir 1977) (for details of the collagen species, see Box 5.2).
5.2.2.1 Box 5.2 Collagen Types Present in the Intervertebral Disc
Fibril forming |
Type I (COL1 α1, COL1α2) |
Type II (COL2α1) |
Type III |
Type V (COL5α1, COL5α2, COL5α3) |
FACIT (fibril-associated collagen with interrupted triple helices) |
Type IX (COL9α1, COL9α2, COL9α3) |
Type XII (COL12α1) |
Basement membrane (basal membrane) |
Type IV (COL4α1, COL4α2, COL4α3, COL4α4, COL4α5, COL4α6) |
Multiplexin |
None |
Other |
Type VI (COL6α1,COL6α2, COL6α3, COL6α4) |
Type X (COL10α1, COL10α2, COL10α3) |
Type XI (COL11α1, COL11α2) |
5.2.2.2 Collagen I
The structure of procollagen I is similar to other fibrillar collagens, and it comprises three polypeptide α-chains, which form a unique triple-helical structure (Fig. 5.2). It is a heterotrimer of two α1 and one α2 chains. It contains an uninterrupted triple-helical domain flanked by short non-helical telopeptides. The telopeptides, which do not have a repeating Gly–X–Y structure and do not adopt a triple-helical conformation, account for 2 % of the molecule. Many macromolecules such as COMP, fibromodulin, matrilin, and decorin attach to collagen I (Fig. 5.3). Collagen I molecules form D-periodic (D = 67 nm, the characteristic axial periodicity of collagen) cross-striated fibrils in the extracellular space, giving the tissue its mechanical strength and providing the major biomechanical scaffold for cell attachment and anchorage of macromolecules (Fig. 5.4).
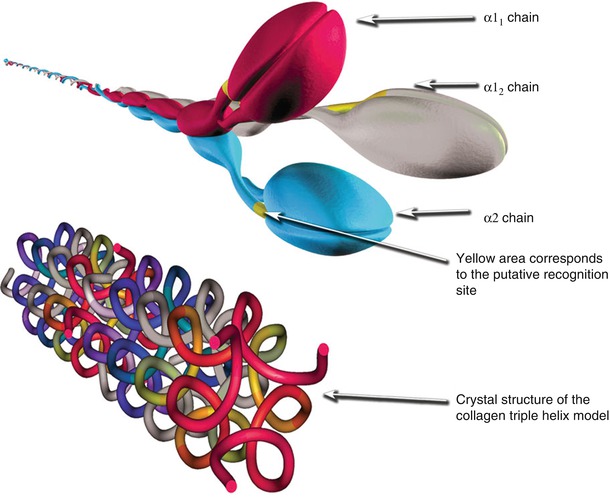
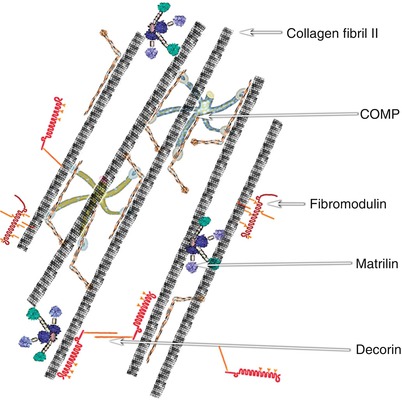
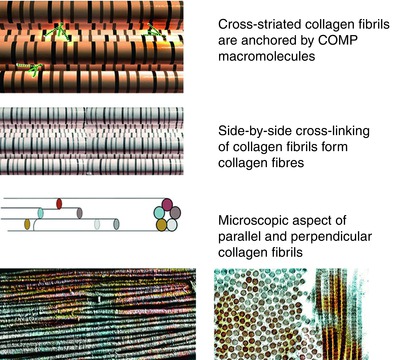

Fig. 5.2
Schematic illustration of type 1 collagen α-chains, recognition site in the C-NC domain, and the crystal structure of the collagen intertwined α-chains (the upper image was generated in 3D Studio Max, while the lower image was generated in CN3D ver. 4.3 using data for Crystal Structure of the Collagen Triple Helix Model [(Pro-Pro-Gly)10]3 from the Protein Data Bank rcsb.org DOI:10.2210/pdb1k6f/pdb)

Fig. 5.3
Schematic illustration of assemblies of cross-striated collagen fibrils and cartilage oligomeric matrix protein (COMP), fibromodulin, matrilin, and decorin in the intervertebral disc (The figure was modified with permission from Feng et al. (2006))

Fig. 5.4
Computer visualization model, schematics, and simulated microscopic images of normal collagen fibrils showing the association with COMP, side-by-side cross-linking of collagen fibrils, and microscopic aspects of collagen fibrils
5.2.2.3 Collagen II
Collagen II fibrils in the nucleus pulposus appear to be organized randomly (Fig. 5.1). In the adult, these collagen molecules are joined by hydroxypyridium cross-links (Eyre and Muir 1976). There is evidence that procollagen II can be expressed in two forms by alternative splicing of the primary gene transcript. The two mRNAs either include (IIA) or exclude (IIB) exon 2, so that procollagen II can be synthesized with or without exon 2 encoding the major portion of the amino propeptide (Sandell et al. 1991; Ryan and Sandell 1990). This polymorphism is thought to influence cell morphology, with the cells expressing procollagen IIA being narrow, elongated, and “fibroblastic” in appearance, while the cells expressing procollagen IIB are large and round. The expression of procollagen IIB appears to be correlated with abundant synthesis and accumulation of aggrecan (Sandell et al. 1991). Procollagen IIA may function in the annulus fibrosus and nucleus pulposus, particularly during development.
5.2.2.4 Collagen VI
Collagen VI accounts for 10–20 % of the total collagen and is particularly prominent in the nucleus pulposus and the end plate cartilage (Roberts et al. 1991). It resembles collagens IX and X in that it is a shorter chain collagen than collagen II. It consists of α-chains that form a highly branched filamentous network based on the formation of tetramers but does not appear to form cross-links with other matrix molecules (Wu et al. 1987). The beaded filaments of collagen VI represent another important network in the disc matrix (Feng et al. 2006). It forms a tetramer of two pairs of antiparallel collagen VI molecules arranged such that two N-terminal ends are exposed at either end of the unit. Further assembly occurs both by end-to-end and side-to-side associations catalyzed by proteoglycans such as biglycan and decorin. These ligands (collagen VI tetramers) are also bound to molecules such as PRELP, fibronectin, and matrilin-1, -2, or -3, which in turn are bound to a collagen fiber, a procollagen molecule, or aggrecan.
5.2.2.5 Collagen IX
Collagen IX is a “fibril-associated collagen with interrupted triple helices” (FACIT) collagen. Other FACIT members are collagen XII, XIV, XVI, and XXI; when compared with collagen IX, they are less characterized in terms of structure and function. Collagen IX is usually found in tissues containing collagen II. It is extensively cross-linked to fibrils of collagen II in an antiparallel orientation and may also be cross-linked to other collagen IX molecules (Eyre et al. 1988; van der Rest and Mayne 1988; Wu et al. 1992). It has a periodic distribution along the fibril of approximately 67 nm (Vaughan et al. 1988). The globular NC4 domain at the N-terminus of the α1(IX) chain extends out from the fibril (Vaughan et al. 1988) and may provide a molecular link between the fibrils and other interfibrillar matrix components, such as the proteoglycan aggrecan. The amino-terminal NC4 domain is very basic (Vasios et al. 1988; Muragaki et al. 1990). Some forms of collagen IX may lack the NC4 domain due to the use of an alternative promoter (Nishimura et al. 1989), and the expression of this variant is thought to be tissue specific and developmentally regulated. Collagen IX of the disc is distinct from that of hyaline cartilage in that the disc contains only the short form of α1(IX) that lacks the NC4 domain (Wu and Eyre 2003). Usage of the short α1(IX) transcript in disc tissue has no apparent effect on cross-linking behavior. The precise biological role(s) of collagen IX, its assembly in relationship to collagen II, and the mechanisms that regulate its synthesis and degradation remain unknown.
5.2.2.6 Collagen X
Collagen X is a non-fibrillar short-chain protein containing three identical α1 chains [α1(X)3] with a low molecular weight of 59 kDa (Schmid and Linsenmayer 1985). Two exons encode the complete primary translation product, which contains the N-terminal region (NC2). The protein is comprised of 52 amino acids, 18 of which form the signal peptide (LuValle et al. 1988). The collagen X molecule also contains a relatively larger (162 amino acids) noncollagenous C-terminal domain (NC1) (Yamaguchi et al. 1989), which plays a key role in the intracellular assembly of the triple-helical collagen X molecules and may be necessary for aggregation to form extracellular networks. The presence of several functional RUNX2 binding sites within the promoter region of the COL10A1 genes suggests that it is a direct transcriptional target of RUNX2 during chondrogenesis (Zheng et al. 2003). It forms pericellular mats and is also closely associated with the fibrils of collagen II (Linsenmayer et al. 1998).
Collagen X is synthesized by hypertrophic chondrocytes during endochondral ossification in the growth plate (Mwale et al. 2000; Tchetina et al. 2003). It was shown to be present in human lumbar discs during aging and degeneration by Boos et al. (1997) and Xi et al. (2004). In addition, nucleus pulposus chondrocytes express collagen X in association with advanced age and degenerative disc lesions (Nerlich et al. 1997; Hristova et al. 2011). Aigner et al. (1998) studied the variation in the pattern of collagen X expression with age in normal human discs, particularly the cells of the inner annulus fibrosus and the nucleus pulposus. These workers showed that with age some cells from the inner annulus or the nucleus expressed a hypertrophic chondrocyte phenotype and secreted collagen X as a component of the disc matrix.
5.2.3 Other Matrix Molecules
5.2.3.1 Cartilage Oligomeric Matrix Protein (COMP)
COMP is a 524-kD protein composed of five identical glycoprotein subunits each of 100–120 kD held together by a five-stranded coiled-coil domain in the N-terminal portion and exposing unique C-terminal globular domains. Each subunit has EGF-like and calcium-binding (thrombospondin-like) domains. It is present in the extracellular matrix of the nucleus pulposus and annulus fibrosus (Ishii et al. 2006; Lee et al. 2007). It exhibits a lamellar distribution pattern in the annulus fibrosus region. Higher nucleus pulposus levels of COMP are found in aging discs (Lee et al. 2007). COMP plays a role in regulating collagen fibril assembly.
5.2.3.2 Fibronectin
Synthesis of fibronectin has been demonstrated in healthy disc tissue (Hayes et al. 2001; Anderson et al. 2010) and cartilage (Wurster and Lust 1984). Fibronectin is a minor component of the disc of young and older animals (Hayes et al. 2001). Its function in the discs is unknown, although it is well established that fibronectin can play a role in cell–matrix, matrix–matrix interactions as well as binding collagen and heparan sulfate proteoglycans through RGD sequences. As a result of alternative splicing, different isoforms of fibronectin exist. Disc cells synthesize fibronectin with either or both the ED-B and ED-A domains present (Anderson et al. 2010), the significance of which is unclear. Fibronectin is elevated in degenerated discs, and its fragments induce the cell to degrade the matrix (Oegema et al. 2000, Anderson et al. 2004).
5.2.3.3 Amyloid
Amyloid deposits in the intervertebral disc were first described by Bywaters and Dorling (1970). In the annulus, amyloid deposits are found lying between thick bundles of collagen fibers, while a diffuse nodular distribution is found in the nucleus pulposus, in proximity to cells (Ladefoged 1985). The incidence of amyloid deposition in discs increases with advancing age, although it is also found in the young disc (Ladefoged 1985; Yasuma et al. 1992). Amyloid deposits of different morphological types have been observed in the disc (Mihara et al. 1994). At the ultrastructu ral level, amyloid deposits are seen composed of 10-nm-wide nonbranching fibrils (Mihara et al. 1994). Changes in matrix glycosaminoglycans, particularly strongly sulfated glycosaminoglycans such as keratan sulfate, may play a role in the pathogenesis of localized and systemic amyloid deposition (Athanasou et al. 1995).
5.2.3.4 Tenascin
Tenascin has also been called hexabrachion (Erickson and Inglesias 1984) and myotendinous antigen (Chiquet and Fambrough 1984). The tenascins are a family of extracellular matrix glycoproteins with repeated structural domains homologous to epidermal growth factor (EGF), fibronectin type III, and the fibrinogens. It has been shown that aggrecan can interact with certain matrix proteins containing EGF-repeats, including tenascins, the fibrillins, as well as the fibulins, (Day et al. 2004) to form higher order networks. Tenascin is a large extended “octopus”-like molecule, in which six arms radiate out from a central point, with each arm having an Mr of 200 kDa and composed of a single polypeptide chain. The domain structure of the cloned molecule supports the finding that it can interact with multiple ligands (Nies et al. 1991). It is a hemagglutinin and can bind, directly or indirectly, with a variety of disc matrix molecules. In discs, it is present in young and adult annulus fibrosus and nucleus pulposus, where it is confined to the pericellular matrix (Gruber et al. 2002, 2006) and its expression can be modulated by mechanical strain (Benjamin and Ralphs 2004). It is thought to have a role in disc aging and degeneration, possibly by modulating fibronectin cell interactions and causing alterations in the shape of disc cells (Gruber et al. 2002).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








