CHAPTER 90 Spinal Intradural Infections
Bacterial Pathogens
Spinal Subdural Empyema
Background
The current understanding of the pathophysiology, diagnosis, treatment, and prognosis of spinal subdural empyemas (SSEs) is based on anecdotal evidence (case reports and case series). It is the rarity of the condition that has necessitated this anecdotal approach. Since the initial description by Sittig in 1927,1 a total of 50 cases have been published.1–39 The exact incidence, therefore, is unknown.
The demographics of the reported cases indicate a slight female predominance, with 27 females and 19 males.36–39 The age at presentation ranges from 9 months to 77 years.35 Approximately 50% of the patients, however, fall into the fifth to seventh decades of life.35 The predominant location of these abscesses tends to be the thoracolumbar spine, with only 10 of the 50 cases being isolated to the cervical spine.35–39
Pathophysiology
Several theories have been proposed to account for the relative infrequency of SSEs when compared with either cranial subdural empyemas or spinal epidural abscesses (SEAs). The absence of air sinuses in the spine may be one of the main factors. Another is the presence of a true epidural space rather than a potential space (cranial) that acts as a filter protecting the subdural space. This accounts for the greater incidence of SEAs as compared with SSEs.40,41 The final theory is the pattern of blood flow of the spine as compared with the brain. The blood is directed centripetally in the spine, whereas it is directed centrifugally in the brain. This again increases the incidence of SEAs when compared with SSEs.20,23,35
The pathogenesis of these infections can be categorized into one of four major categories. The first and most common mechanism for the development of an SSE is hematogenous spread from a distant source.* Although the primary site of infection may be anywhere, Bartels and colleagues35 found the most common site to be peripheral infections such as furuncles and cellulitis. Other primary infections include respiratory tract infections, endocarditis, urinary tract infections, and septic abortions.35,36 The next most common source is iatrogenic. This includes lumbar punctures, local anesthetic injections, and discography.* A third category comprises all infections arising from direct extension into the subdural space. These infections may be due to dermal sinus tracts associated with spinal dysraphism,4,21,23,39 spinal infections,35 or trauma to the spine.11 The fourth major category is an unknown primary source.11,13,20 Bartels and colleagues35 found that the patients in 10 of 45 cases reviewed had no known primary source for their infections.
The organisms responsible for SSEs reflect the primary sites of infection. It is not surprising, therefore, that the most frequently cultured organism is Staphylococcus aureus. Of the total of 50 reported cases, 24 have been due to S. aureus.† The remainder of cases can be attributed to a variety of organisms including other Staphylococcus species,13,25,30 Streptococcus,8 Escherichia coli,35,39 Pseudomonas aeruginosa,16 Streptococcus pneumoniae,14 and Peptococcus magnus (Box 90–1).21
Clinical Presentation
†References 1–3, 5, 7–9, 11, 12, 15, 18, 19, 22, 23, 28, 29, 36–38.
Fraser and colleagues15 described the classical presentation of an SSE in 1973. The triad includes fever and neck/back pain followed by symptoms of spinal cord/cauda equina compression. Levy and colleagues36 found the triad was present in 18 of 47 cases they reviewed. In their review, Bartels and colleagues35 confirmed the progression of symptoms as described by Fraser and associates. They found that at the time of initial onset of symptoms, 84.4% had spinal/limb pain and 55.6% had fever.35 By the time the patients had reached medical attention, 86.7% had fever, 84.4% had spinal/limb pain, and 82.2% had a motor deficit.35 A key feature of SSE is the absence of spinal tenderness, which helps distinguish this process from the more common SEA.15,16 However, the presence of spinal tenderness does not preclude the diagnosis of an SSE. Levy and colleagues36 found 14 of the 47 patients they reviewed did have spinal tenderness.
On the basis of their findings, Bartels and colleagues35 proposed a sequence of three stages in the progression of these infections. Stage 1 included fever with or without spinal pain. Stage 2 adds motor, sensory, and/or sphincter disturbances to the picture. Stage 3 is defined as complete motor and sensory loss below the lesion.35 The rate of progression from one stage to the next, however, is variable and unpredictable.
Laboratory Evaluation
The complete laboratory evaluation of patients who present with a clinical picture suggestive of an SSE is essential, although often nonspecific. Serum leukocytosis may be mild to moderate.16,35–39 The erythrocyte sedimentation rate (ESR) may be prolonged,35,36 and the C-reactive protein level may be elevated.39
Another essential part of the workup is obtaining cultures from all sources. This does not include the routine use of a lumbar puncture for obtaining cerebrospinal fluid (CSF) because a lumbar puncture risks contaminating deeper meningeal layers.36 If CSF is obtained, it will often show characteristics of a parameningeal process (not meningitis). This includes moderate pleocytosis, moderately elevated protein content, and low to normal glucose levels. The CSF cultures are generally negative.15,16,19,36,38
Imaging Studies
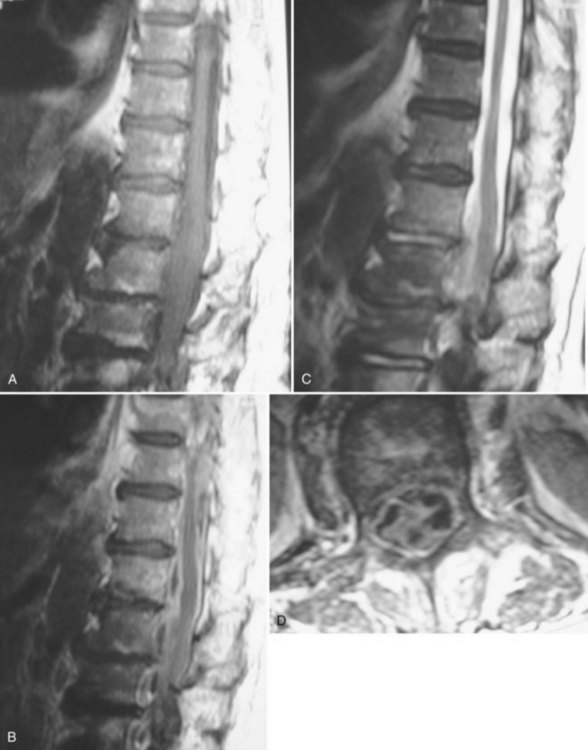
Much of the literature regarding SSEs was published before the routine use of magnetic resonance imaging (MRI) in the evaluation of patients with a clinical presentation suggestive of an SSE. The diagnostic study of choice at that time was a myelogram with or without a postmyelographic computed tomography (CT).1–29,31–35 The current definitive diagnostic study of choice is a contrast medium-enhanced MRI because it is noninvasive and allows better visualization of the spinal cord, vertebrae, disc spaces, extent of lesion, and extent of compression. Because there are only a total of six published cases of the imaging characteristics of SSEs on MRI, the exact findings are not well described.30,36–39 The basic finding, however, is an intraspinal, space-occupying, variably enhancing mass (Fig. 90–1).36–39 The major limitation of MRI, however, remains its inability to distinguish whether the lesion is intradural or extradural.38,42 The differentiation of epidural versus subdural may be aided by an evaluation (using plain radiographs and MRI) for the presence of discitis/osteomyelitis, which accompanies two thirds of SEAs.43
Treatment and Prognosis
The mainstay of treatment for these infections is surgical decompression (laminectomy) with irrigation and drainage of the subdural space followed by appropriate antibiotic therapy.35,36,44 The exposure should encompass the extent of the abscess.44 After copious irrigation, most authors advocate the primary closure of the dura. The arachnoid should be preserved if possible.36,44 Although some have advocated using postoperative irrigation via external drains, this has not been adopted as part of the conventional treatment algorithm.18 A significant indication for surgery is obtaining a definitive organism to treat; therefore cultures should be obtained before using antibiotic irrigation.
The use of postoperative antibiotics should be based on the given organism found during surgery. Empiric antibiotic coverage for these infections must cover gram-positive cocci.36 Some advocate the additional use of corticosteroids (dexamethasone) during the perioperative period as a prophylaxis against the development of thrombophlebitis.15,36 This is another technique that is not universally applied.35,37–39,44
The prognosis for patients with SSE is directly related to the treatment methodology.35,36 Although some have had success with conservative treatment,37 the overall prognosis is much better if patients undergo aggressive irrigation and drainage.35 In a review of 45 patients by Bartels and colleagues,35 the outcomes correlated with the treatment approaches. Among the surgically treated group, 82.1% made a complete recovery or improved, whereas 17.9% died. In the conservatively treated group, 80% died (four of five patients) and only 20% (one of five patients) improved.35 On the basis of these numbers, the current recommendations are for aggressive surgical treatment followed by antibiotic therapy.43,44
Spinal Intramedullary Abscess
Background and Demographics
Intramedullary spinal cord abscesses (ISCAs) are also an uncommon condition. Since the initial case description was published by Hart in 1830, 96 total cases have been published45–91 Although the exact incidence is unknown, an autopsy study conducted by Courville in 1950 found only one spinal cord abscess among 40,000 postmortem examinations.92 As with SSE, much of the current understanding regarding intramedullary spinal cord abscesses is based on case reports and several retrospective reviews of these published cases. Arzt46 reviewed all 42 published cases reported in the preantibiotic era between 1830 and 1944. In 1977 Menezes and colleagues and DiTullio47,48 independently reviewed all published cases of ISCA between 1944 and 1977. Most recently, Bartels and colleagues,72 as well as Chan and Gold,78 have published reviews encompassing the cases described since 1977.
ISCA is a disease that commonly afflicts children. In reviewing patients presenting either before or after the discovery of antibiotics, there is a consistent plurality of patients (40%) presenting at younger than 20 years of age.47,48,72,78 In fact, 25% to 27% of patients are younger than the age of 10.47,48 The age range, however, is quite broad and extends from 7 months to 72 years.47,48,72,78 There is a male predilection, with 60% to 70% of cases occurring in men.48,72
ISCAs occur throughout the spine, but they are most frequently found in the thoracic region.47,48,72,78 Bartels and colleagues72 found in their review that 32% of lesions were isolated to the thoracic cord, as compared with 17% and 12% in the cervical and lumbar regions, respectively. Overall, 69% of all abscesses involved some portion of the thoracic cord.72 Six cases of holocord abscesses have also been published.81
Pathophysiology
The pathogenesis of ISCA can be divided into two broad categories: direct implantation and hematogenous spread. The more complex of the two is hematogenous spread via arterial supply, venous drainage, or lymphatics. The venous system that drains the spinal cord is a low-pressure system that communicates with the venous drainage of the chest and abdomen. Changes in the intrathoracic or abdominal pressures may generate backflow in these veins and allow seeding of the spinal cord with an infectious embolus from the thorax or abdomen.48,93
Arterial metastasis of an infection to the spinal cord is another route for developing ISCA. An understanding of the pathophysiology of this process can be derived from experimental work done by Hoche.94 He found that transient bacteremia generated by injecting various organisms into the arterial supply of the brain or spinal cord was not sufficient for the production of a parenchymal abscess. However, injection of an aseptic embolus followed by an injection of bacteria into the arterial supply of the brain or spinal cord did result in the formation of an abscess. In addition, injection of septic emboli also formed abscesses. The basic finding is that metastatic abscesses form in the central nervous system (CNS) as a result of septic emboli or bacterial infection of an area that was previously infarcted by an aseptic embolus.48,94
Menezes and colleagues48 describe a third route for the formation of metastatic abscess in the spinal cord. On the basis of experiments conducted by Galkin in the 1930s, the Virchow-Robin spaces of the spinal cord have connections with the lymphatics that drain the mediastinum, abdomen, and retroperitoneum.95 This connection is the lymphatic channels found along the spinal nerves. It is, therefore, theoretically possible for infections of the chest and abdomen to reach the spinal cord simply through their lymphatic drainage.48
The site of origin for cases of metastatic ISCA is variable. The most common primary site is pulmonary, owing to conditions such as pneumonia and bronchitis.47,48,66,72,78 Other primary infections include endocarditis,46–48 urinary tract infections,57 peritonitis,90 and peripheral skin infections.48,72,76
The other major route for the pathogenesis of ISCA is direct implantation. The most common source of direct implantation/contiguous spread is via a congenital midline neuroectodermal defect.52,55,59,60,69 Chan and Gold found that among the 25 cases they reviewed, 24% were a result of contiguous spread from a dermal sinus tract.78 The other sources include postoperative,64 post-traumatic (stab wound),48 and postprocedural infections.48
The most common mechanism for the development of ISCA, however, has become cryptogenic. In their review of 25 cases reported between 1977 and 1997, Chan and Gold78 found that 16 cases (64%) were cryptogenic. In Menezes’48 review, the cause of infection was unknown in 50% of cases reported after 1960. The case reports published since Chan and Gold’s series also support the observation that cryptogenic ISCAs are a highly frequent finding.77,81,85,87,88
Chan and Gold78 also compared their review of 25 patients treated in the antibiotic era (1977-1997) with the 42 patients reported in the preantibiotic era (1830-1944) and reviewed by Arzt. Several trends were elucidated by this comparison. One, highlighted earlier, is the increased frequency of cases with a cryptogenic mechanism. Another is the dramatic drop in the number of cases caused by hematogenous spread. In the preantibiotic era, cases of ISCA were far more frequently caused by hematogenous spread from an extraspinal source than during the antibiotic era (45% vs. 8% of cases).78 This trend may be attributed to the effectiveness of antibiotics in treating primary infections.
The organisms responsible for ISCA reflect the sources of the infection. In their review of 93 reported cases of ISCA, Bartels and colleagues72 found that a causative organism was reported in 56 cases. Among these, Staphylococcus accounted for 22 cases and Streptococcus was found in another 16 cases.72 The other significant organisms were Actinomyces, Proteus mirabilis, Pneumococcus, Listeria monocytogenes, Hemophilus, and Escherichia coli (Box 90–2).48,56,72,77,78
The organisms responsible for ISCAs can be further divided on the basis of the mechanism of formation for the given abscess. Cases of contiguous spread via a dermal sinus tract are most commonly due to †References 48, 55, 56, 63, 67, 73–75, 77, 78, 85.
Staphylococcus epidermidis, S. aureus, Enterobacteriaceae, anaerobes, and Proteus mirabilis.* Postsurgical (contiguous) cases are most often due to S. epidermidis, S. aureus, Enterobacteriaceae, and Pseudomonas aeruginosa.78 The cases that arise from hematogenous spread reflect the site of primary infection. The organisms found most often in cases that have a cryptogenic etiology include Listeria monocytogenes, Streptococcus viridans, Actinomyces meyeri, and Hemophilus species.† The high frequency of L. monocytogenes is based on the bacteria’s trophism for the CNS.78 The other major organisms are oral flora and may lead to ISCA after bacteremia from an odontogenic source.78
The histopathology of ISCA follows the same basic pattern as cerebral abscesses. The early stages of acute abscess formation appear microscopically as nodules composed of monocytes, lymphocytes, polymorphonuclear leukocytes, and endothelial cells. These lesions are often in proximity to vessels. There are organisms in both the abscess and the associated vessel. Areas of hemorrhage often surround these septic nodules. Veins in the area of the abscess are frequently thrombosed. As these lesions grow larger, they begin to develop purulent myelitis with areas of central necrosis. The chronic abscesses have a well-defined three-layer capsule surrounding a central area of pus and necrosis. The inner layer is composed of collagen fibers and polymorphonuclear leukocytes. The middle layer contains fibroblasts, capillaries, histiocytes, and plasma cells. The outer layer is essentially connective tissue.48,76,78 The growth of ISCAs begins in the gray matter of the spinal cord. It then extends rostrad and caudad along fiber tracts.76
Clinical Presentation
The presenting signs and symptoms in patients with ISCAs almost always involve motor deficits, which vary depending on the location of the abscess. At the time of diagnosis, 83% to 94% of patients will have some type of motor deficit.47,72,78 The extent of the deficits is variable from slight paresis to complete paralysis.72 Sensory disturbances are almost as frequent as motor findings, with 60% to 78% of patients having some degree of sensory loss before treatment.42,72,78 Although it does not appear as early as motor and sensory disturbances, 51% to 56% of patients will have loss of sphincter control at the time of diagnosis.72,78
The other two commonly encountered signs are spinal pain localized to the involved area and fever. The percentage of patients who are febrile at the time of their diagnosis ranges from 25% to 50%. The presence of spinal pain at some point before diagnosis occurs in 36% to 60% of patients.71,72,78 Less commonly found symptoms include Horner syndrome,56,76 brainstem findings,85 and Brown-Séquard syndrome.90
Menezes and colleagues48 divided the ISCAs into three clinical categories on the basis of symptomatology and chronicity. Acute infections are defined as infections with symptoms lasting less than 2 weeks. The symptoms in this group consist of complete or partial transverse myelitis commonly associated with fever and leukocytosis.48,76 The subacute lesions are defined as having a clinical history longer than 2 weeks but shorter than 6 weeks. The symptoms in this category consist of a stuttering onset of spinal cord dysfunction similar to the presentation of intramedullary tumors. Chronic abscesses are those with a clinical history longer than 6 weeks. These chronic ISCAs also present with symptoms that mimic the presentation of an intramedullary tumor. These patients rarely have fever or leukocytosis at presentation.48,76
Laboratory Evaluation
Laboratory studies are not consistently abnormal in patients with ISCAs. Leukocytosis, although more common in cases with acute presentation,76,81,85,87 may be present in chronic cases.48,76 It may also be absent in both acute56,72 and chronic cases.77,88,90 An abnormally prolonged ESR and elevated C-reactive protein levels are also inconsistent findings.76,77,81,88 The analysis of the CSF is not consistent, either. The one consistent finding is that CSF cultures are routinely negative.* If the CSF is abnormal, the profile reflects the presence of a parameningeal process with elevated white blood cells (WBCs), mildly elevated protein count, and normal glucose levels.48,56,71,72,76,90
Imaging Studies
Plain radiographs, myelograms, CT scans, and MRI have all been used to evaluate patients with ISCAs. Plain radiographs are almost always normal.53,71 The utility of plain radiographs is in evaluating for the presence of osteomyelitis, spinal deformity, spinal stenosis, and spinal dysraphism, which have all been shown to be predisposing factors for the development of ISCA.78 Myelography was the diagnostic study of choice before the routine availability of MRI. The findings on myelogram include symmetric or asymmetric widening of the spinal cord at a focal segment, partial or complete obstruction to flow, and low-lying conus/tethered spinal cord (spinal dysraphism).48,56,72,78 The use of CT of the spine was often in conjunction with a myelogram. Postmyelographic CT may show segmental widening of the spinal cord and/or partial or complete obstruction to CSF flow in approximately 60% of cases.78 CT done without intrathecal contrast medium enhancement may show widening of the cord, and some scans done with enhancement reveal an intramedullary process.72,76
The current diagnostic method of choice, however, is an enhanced MRI. The imaging of ISCA follows essentially the same progression that has been documented in the development of brain abscesses. The two main stages are early and late myelitis.96 Early myelitis shows up as hyperintense signal on T2-weighted and proton density-weighted sequences. The T1-weighted sequences show isointense to hypointense signal changes with a widened spinal cord. There is poor contrast medium enhancement on T1-weighted sequences during the early myelitis phase. The T2-weighted hyperintense signal changes are more diffuse and extensive than the signal changes on the T1-weighted sequences. The late myelitis stage corresponds to the pathologic stage of capsular formation. At this point there is more clearly defined marginal enhancement on contrast medium-enhanced T1-weighted images. Generally, the well-defined enhancement classically described in abscess formation is not seen until 7 days after initial presentation. The T2-weighted hyperintense signal changes become less diffuse during the late myelitis phase.71,72,76,78,81,85,88
The resolution of abnormalities on MRI is variable. With treatment, the T2-weighted hyperintense signal changes resolve over several weeks. The resolution of the T1-weighted contrast medium enhancement takes several months.96
Treatment and Prognosis
Several factors have been found to be prognostically significant. Menezes and colleagues,48 in their review of the 55 cases described between 1830 and 1977, found that prognosis was linked to the clinical presentation. Patients presenting with acute onset of symptoms had a 90% mortality rate as compared with 66% for subacute presentations and 53% for chronic presentations.48 The confounding factor within this group, however, is the use of antibiotics. Among the 55 cases reviewed, 17 were treated during the antibiotic era (after 1944). The mortality rate within this group was 23%.48 Chan and Gold corroborated this finding in their review. They found the mortality rate among patients treated without antibiotics (1830-1944) was 90% as compared with 8% among those treated with antibiotics (1977-1997).78 Aggressive treatment with antibiotics requires empirical therapy until an organism has been isolated. The choice of antibiotics for empirical therapy should be based on the suspected source of infection and then adjusted on the basis of the operative culture results.48,71,72,78 The exact duration of antibiotic therapy has not been well defined. The current recommendation is a minimum of 4 to 6 weeks of parenteral therapy.48,78,85
Despite the confounding effects of antibiotic therapy, the chronicity of the patient’s symptoms does appear to be a prognostic factor. Other studies indicate that patients presenting with acute symptoms have a worse prognosis in terms of neurologic recovery.71
Although case reports do exist of patients treated successfully without surgical decompression and drainage,90 the current recommendations are for immediate surgical treatment.47,48,71,72,78 The surgery should include laminectomies at the involved levels, intradural exploration, midline myelotomy, and irrigation and drainage of the abscess cavity. Prompt surgical drainage has a significant effect on neurologic recovery and mortality. In their review of 93 cases, Bartels and colleagues72 found that the mortality among patients treated nonoperatively was 100%. In contrast, surgically treated patients improved or had a complete recovery in 77.9% of cases. One case (1.7%) worsened after surgery, 4 (6.8%) were unchanged, and in 8 (13.6%) the patients died after surgery. The modern surgical outcomes are even better because 6 of the 8 patients who died did not receive antibiotics after surgery.72 Overall, the death of a patient diagnosed with an ISCA is most frequently due to the presence of multiple CNS abscesses and, specifically, to brain or brainstem abscesses.48,66,67,78
Mycobacterial Pathogens
Spinal Intradural Tuberculosis
Background
The initial description of spinal tuberculosis was written by Percival Pott in 1779.97 It included the surgical drainage of a spinal abscess for the treatment of paraplegia. Despite this initial surgical approach, the mainstay of treatment remained rest (in a sanatorium), nutrition, and fresh air until the discovery of antituberculosis drugs in 1944. Chemotherapeutic agents dramatically reduced the incidence and improved the prognosis of tuberculosis. The role of surgery, however, has always been a significant part of the management of spinal tuberculosis.
The term Pott disease refers to tuberculous spondylitis with any associated epidural extension but does not include intradural tuberculosis. There are four major classifications for intradural spinal tuberculosis. These include subdural tuberculomas, meningitis, arachnoiditis, and intramedullary tuberculomas. The most common type of neurotuberculosis, tuberculous meningitis, is not discussed in this chapter because it is a diffuse CNS pathologic process that presents mainly as brain and cranial nerve dysfunction.98 The initial description of a spinal intradural lesion caused by tuberculosis was published by Abercrombie in 1828.99 He specifically described the case of an intramedullary tuberculoma.
Epidemiology
In the latter half of the 20th century, the incidence of tuberculosis in developed countries steadily declined with the use of antituberculosis drugs. Beginning in 1985, however, the incidence began to rise secondary to the spread of human immunodeficiency virus/acute immunodeficiency syndrome (HIV/AIDS).100,101 The demographics of tuberculosis can still be divided between developing and developed countries.97,102–104 In developing countries with higher disease prevalence, the disease commonly affects children and young adults. It also tends to be more aggressive. In developed countries, the disease tends to afflict older individuals, as well as those with HIV/AIDS and recent immigrants.97,104,105
Pott’s disease occurs in less than 1% of patients with tuberculosis.106 It accounts for 50% of musculoskeletal tuberculosis.105 CNS involvement is even less common. Bucy and Oberhill107 found evidence of CNS tuberculomas in 1 of every 53 cases of tuberculosis. In their review of 38,510 patients treated for tuberculosis between 1935 and 1957, Arseni and Samitca found CNS tuberculomas in 210 cases. Only five cases were intramedullary tuberculomas, and another three were intradural extramedullary tuberculomas.108
The rarity of intradural infections is also exemplified by the fact that since the initial description by Abercrombie in 1828 approximately 178 cases of intramedullary tuberculomas of the spine have been reported. These have been compiled in three major reviews. Lin published a review in 1960 of 105 cases reported between 1828 and 1960.109 MacDonell and colleagues104 reviewed another 42 cases reported between 1960 and 1990. In the most recent review, Ratliff and colleagues110 compiled 31 cases published between 1990 and 1999.
The majority of these cases come from developing countries. In MacDonell and coworkers’104 study only 4 of 43 cases come from the United States and Italy. The remaining 39 come from India (26 cases), Morocco, Sri Lanka, and other developing nations.104 This distribution is also echoed in the work of Ratliff and colleagues.110 The average age at presentation in MacDonell and colleagues’104 study was 28.6 years. The average age in those cases from developing countries is younger (24.9 years), which corresponds to the more aggressive nature of infection in these regions.104,111–115 The average age in cases from developed countries is older (47.8 years).104,110,116,117
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree