68 Spinal cord stimulation (SCS) is a highly versatile method of treating neuropathic pain that involves delivering therapeutic doses of electrical current from the epidural space into the spinal column to several spinal cord structures, including the dorsal column–medial lemniscal afferent pathway. Electrical stimulation of the large myelinated Aβ afferent fibers involved in the dorsal column system results in ipsilateral tingling paresthesia and causes decreased discomfort and pain sensations. While the mechanisms of SCS are still unclear, leading theories involve the generation of complex electric fields in the epidural space affecting the activity of a large number of structures, including both descending and ascending pathways, which may increase central pain modulation from higher-order central nervous system (CNS) structures. Although SCS may be used to treat many forms of intractable pain, it is most commonly indicated to treat postlaminectomy syndrome, otherwise known as failed back surgery syndrome (FBSS), and complex regional pain syndrome. This chapter focuses on the techniques and issues involved in treating back pain, particularly FBSS, using SCS. FBSS is a vaguely defined syndrome that has been used to classify chronic pain localized to the lower lumbar area, pain in the buttocks, persistent radicular pain, or diffuse pain of the lower extremities that occurs after one or more lumbosacral surgeries. Because FBSS is classified by back and leg pain, current literature on SCS efficacy has started to distinguish the locations of pain. While SCS is commonly used to treat low back pain, it has been shown to be more effective in relieving pain in the lower limbs. Common problems that FBSS may be indicative of are broad and include arachnoiditis, epidural fibrosis, radiculitis, microinstability, recurrent disk herniations, and infection. SCS is best used for neuropathic pain, as opposed to nociceptive pain being induced by physical findings within the spinal column. Patients suspected of having FBSS present with chronic back pain of neuropathic origin, require analgesics, and are unable to return to work following at least one prior spinal surgery. Obtaining detailed information about the patient’s current symptoms is vital to determining the method of treatment that can be used for FBSS. During the history, the examiner should ask specific questions to assess for mechanically induced symptoms. Are the symptoms different or worse with sitting versus standing and relieved with lying down? Do symptoms improve with traction, stretch, or the buoyancy of a pool? Is there a particular sensitivity to pain, implying neuropathic symptoms over nociceptive or mechanical stresses? Understanding the exact distribution of the back pain is paramount. Many patients may describe the gluteal region as the “back.” “Sacroiliac joint pain,” “hip pain,” and “mid and low back pain” are descriptions in the back, and the response to stimulation varies. SCS therapy is dependent on producing a paresthesia sensation over the painful area. Typically, it is possible to deliver this paresthesia in the gluteal region; however, delivery of the stimulation in the sacroiliac region or to the mid-back above the iliac crest is often difficult. Other valuable diagnostic information includes the extent of pain relief achieved by surgery and the length of postoperative time in which the patient was pain free. In some patients, careful questioning will reveal that the pre-operative symptoms were never relieved with surgery, and this may implicate neuropathic pain from the onset. Careful psychological screening is important in determining candidacy for SCS, because mood disorders can alter pain perception and reporting; in addition, other psychiatric diseases, major depression, anger, or unrealistic expectations can make patients inappropriate candidates for treatment. If patients receive appropriate treatment for such psychiatric disorders, they may subsequently become candidates for SCS. A thorough neurological exam should be conducted to identify any neurological deficits that may be present, which may be attributable to a structural lesion and, therefore, could be otherwise addressed with surgical decompression. As with the evaluation of any patient with low back pain, these symptoms should be correlated to imaging studies before making a clinical decision. Gait, spinal alignment, range of motion, sensation, and strength must all be examined. As mentioned previously, complete and appropriate imaging studies, including a computed tomography (CT) scan or magnetic resonance imaging (MRI), may be performed to rule out a structural etiology of the patient’s pain. Specifically, an MRI with and without intravenous gadolinium should be obtained. Currently, there are two basic types of electrodes used for SCS stimulation: percutaneous leads and paddle electrodes. Percutaneous electrodes or wire electrodes are advantageous because they require less dissection for implantation. These electrodes are available with four (quadripolar) or eight (octopolar) contacts of various spacing and length. Percutaneous electrodes are appropriate for testing candidacy for permanent implantation, as they can be advanced over several segments of the spinal cord to determine optimal electrode positioning. Use of electrodes with more contacts and greater spacing allows testing of a broader range of spinal cord segments, while using electrodes with fewer contacts and smaller spacing allows better targeting of segments and electric field shaping. Present strategies involve using one or two quadripolar electrodes for limb pain and one or two octopolar electrodes for axial pain. The efficacy of placing a third electrode to create better steering of electric fields is still unclear, but this strategy has definite theoretical advantages.1,2 Disadvantages to percutaneous electrodes include their tendency to migrate after implantation, given their cylindrical shape and flexibility. Another drawback is decreased energy efficiency of percutaneous leads because the current is distributed circumferentially around the lead, including areas that do not contact the spinal cord. However, there have been recent studies that have indicated that the rate of percutaneous electrode migration may be slightly less than the rate of paddle electrode migration.3 In addition, the same studies have indicated that the rate of breakage of percutaneous electrodes is almost half the rate of breakage of paddle electrodes.3 Paddle electrodes are offered in many sizes, shapes, lengths, spacings, and configurations of electrodes. Currently, single, dual, and three-column configurations are readily available. Recently, one manufacturer has released a five-column paddle. The more complex electrode arrays provide surgeons increased control for shaping the electric field; however, at the same time, increasing the number of contacts on a given array significantly increases power consumption by the system and complexity of programming. In 2005, it was estimated that ~ 3% of implanted SCS paddle leads were three- or five-column leads; however, in 2009 it was estimated that ~ 60% are three- or five-column leads (personal communication from St. Jude Medical Marketing, November 30, 2010) (Table 68.1). Paddle electrodes are advantageous because they are more energy efficient and should be the primary choice in patients who have had previous spinal surgery at the intended level of implantation or surgery below the intended target, preventing access by percutaneous leads. The accumulation of scar tissue following previous spinal surgeries makes it more difficult to place percutaneous electrodes properly with the Touhy needle. In addition, patients with previous laminectomies can have paddle electrodes implanted more readily because the major portion of the procedure has already been performed. Furthermore, some patients do note significant positional and postural changes with percutaneous leads. These patients may benefit from a complex plate lead or increased stiffness of a plate electrode. For this reason, patients who are observed to have higher energy requirements during the percutaneous trial may do better with a permanent paddle instead. A common practice is performing trials with percutaneous leads and reserving paddle leads for those patients undergoing permanent implant after a successful trial.
Spinal Cord Stimulators for Back Pain
![]() Classification
Classification
![]() Workup
Workup
History
Physical Examination
Imaging Studies
![]() Treatment
Treatment
Equipment
Permanent cases | 2007 | 2011 |
1—Column paddle | 12% | 15% |
2—Column paddle | 41% | 7% |
3—Column paddle | 47% | 22% |
5—Column paddle | 0% | 56% |
Total paddle cases | 38% | 53% |
Total percutaneous cases | 62% | 47% |
There are two categories of generators available that can be used to deliver the input to the electrodes for SCS. Radio frequency (RF) generators are now less commonly used and involve transcutaneous transmission of signals from an external transmitter to a subcutaneously implanted receiver. Instead, most practitioners will use implantable pulse generators (IPG), which contain lithium batteries and can be programmed by the physician and adjusted transcutaneously by the patient with a remote control provided. The battery life of this type of device is typically 2.5 to 4.5 years and varies with usage factors, such as voltage, rate, and pulse width. Rechargeable IPG devices have also recently become available and are estimated to have a battery life of 5–9 years. These newer systems may start replacing the nonrechargeable batteries as well, and they may be more cost effective despite the increased initial cost of these devices.4
Placement of the IPG should be performed in an area with sufficient overlying tissue but no underlying bone. In the buttock region, three bony landmarks are used to best position the IPG: the posterior superior iliac crest, the greater trochanter of the femur, and the apex of the iliac crest (Fig. 68.1). The IPG is then placed in the lateral portion of the triangle formed by these three landmarks.
Patient Management/Evaluation
When using SCS to treat FBSS, one of the greatest challenges is to achieve paresthesia in the low back. Often SCS is unable to cover axial back pain located in the mid-back or even in the upper lumbar spine. Instead, the types of axial back pain that can have positive outcomes lower lumbar or gluteal-region pain. Even when direct stimulation of the lower back is achieved, the initial pattern of paresthesia is sometimes replaced by an unpleasant segmental band of stimulation from the thoracic roots over time. Unfortunately, this negates the initial benefits of the procedure. Newer technologies are starting to address this with current steering, as seen with multiarray electrodes.
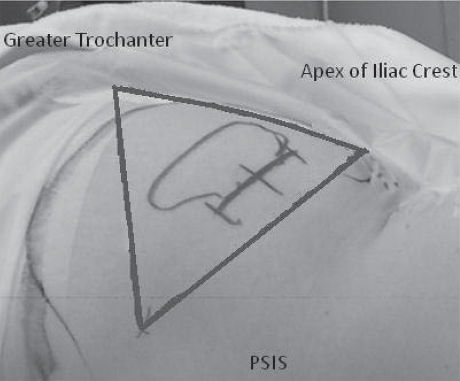
Fig. 68.1 Preoperative photograph depicting ideal placement of current IPGs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






