Chapter 40 SLE in Childhood and Adolescence
Children and adolescents with systemic lupus erythematosus (SLE) represent both a special challenge and a special opportunity. Early onset allows us to observe the natural history of SLE and to investigate potential causes, free from the confounding factors that may be present in older patients.1 Recognition of the special considerations that relate to ongoing physical and emotional growth directly influences the choice of medications and the likelihood of success. A satisfactory outcome for the child with SLE is not simply a 5- or 10-year survival period but a 50- or 60-year survival period.
Compliance is one of the most profound determinants of outcome for SLE. It cannot be assumed that the child and family will comply. Children and adolescents are extremely vulnerable to the psychological impact of both the chronic illness and the medications that dramatically alter their appearance (Figure 40-1). To offset peer group pressures on both the child and the family, which may be overwhelming, excellent medical care must be coupled with multidisciplinary family education and support. Without compliance, even the best therapeutic regimen is ineffective.
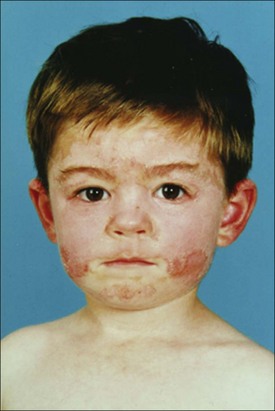
FIGURE 40-1 Altered facial appearance in a young man with systemic lupus erythematosus.
(From Hochberg MC, Silman AJ, Smolen JS, et al. [editors]: Rheumatology, ed 5, Philadelphia, 2010, Mosby.)
Although childhood-onset SLE often is described as more severe and a large proportion of children and adolescents have significant renal or central nervous system (CNS) involvement at the time of diagnosis,2,3 the perception of increased severity may arise from delayed diagnosis and poor compliance.
English-language reports of children with SLE appeared as early as 1892. Sequeira and Balean,4 writing from the London Hospital in 1902, noted that the disease commences early in life in a much larger proportion of patients than was commonly believed. Series of children with SLE began to be published in the 1950s and 1960s. In the era before steroids were available, childhood-onset SLE was a rapidly evolving and usually fatal multisystem disease. SLE is now a common diagnosis in every large pediatric rheumatology program. With proper care, most children and adolescents with SLE now have an excellent prognosis.
Epidemiology
The incidence and prevalence of SLE in childhood have been estimated at 0.5 to 1 in 100,000 and 1 in 10,000, respectively. The influences of sex and racial origin on the occurrence and manifestations of SLE are widely recognized.5 In childhood, the influence of race is striking. The age- and sex-adjusted prevalences of SLE in African American, Asian, and Hispanic children were more than threefold those of Caucasian children at one large center. Although based on a limited sample, these data suggest a significant variation in the influence of sex hormones and puberty on the predisposition to SLE among different races.
Diagnosis
Although physicians often rely on antinuclear antibody (ANA) testing, which is useful for prompting the consideration of SLE, a positive test is not sufficient for the diagnosis. Conversely, ANA-positive children who fulfill at least one other criterion should be periodically reevaluated. Definite SLE may manifest decades after the initial presentation.6
In most ways, diagnosing SLE in childhood is the same as diagnosing SLE in an adult. Confirming the diagnosis of SLE in children and adolescents is based on criteria developed by the American Rheumatism Association (ARA) for use in adults.7 Classification as definite SLE is based on the fulfillment of four criteria, but the diagnosis should not be automatically discarded in children who meet only three. Although the ARA criteria are useful guidelines, the fulfillment of four criteria does not exclude other diagnoses; similarly, the failure to fulfill four criteria does not exclude SLE. It is not unusual for children to be told that they do not have SLE because they fulfill only two or three of the criteria developed by the American College of Rheumatology (ACR) only to be recognized as having definite SLE within an additional 1 to 2 years. In many patients the initial dismissal of the diagnosis results in a delay in further evaluation or the return to medical care despite progressive symptoms. Physicians evaluating children who do not fulfill all the necessary criteria at the time of the initial evaluation must remain aware that additional findings may evolve and counsel families accordingly.
Clinical Manifestations
Unexplained elevated body temperature, malaise, and weight loss are the most common manifestations of SLE in children and adolescents. Because these nonspecific symptoms may be associated with many chronic illnesses, the physician should actively seek evidence of arthritis or a photosensitive rash, hematuria or proteinuria, hypergammaglobulinemia, and hypocomplementemia. Any of these findings should prompt consideration of SLE, but one cannot rely on their presence. On the initial evaluation, the patient and family often do not describe findings such as arthritis of the small joints of the hands, alopecia, or photosensitivity unless they are specifically questioned. The reported frequency of many complaints varies widely among series of children with SLE, reflecting selection and referral criteria and the care with which the complaint was sought (Table 40-1).8
Renal Disease
Renal disease is evident in nearly two thirds of children and adolescents with SLE.2,3,8 Renal manifestations range from mild glomerulitis to sudden renal failure. Hematuria, proteinuria, and hypertension may be present in any combination. In the absence of nephrotic syndrome, renal involvement may be silent in childhood.
Renal biopsy without regard to clinical manifestations demonstrates varying renal involvement in most children.9 Children with a normal urine sediment level typically have only mild glomerulitis. Although occasional biopsies demonstrate silent diffuse proliferative glomerulonephritis (DPGN), the significance of silent DPGN is uncertain. Series reporting follow-up of silent nephritis in SLE describe a benign prognosis.9 Thus the importance of detecting silent DPGN is uncertain. Renal biopsy should be performed if necessary to confirm the diagnosis, to investigate unexplained changes in renal function, and when the clinician is considering or monitoring the effects of aggressive therapy.
Renal involvement is categorized according to criteria developed by the World Health Organization (WHO). Mild glomerulitis is the most benign form, followed by focal segmental glomerulonephritis and membranous glomerulonephritis.10 DPGN carries the greatest risk of chronic renal failure and is the most frequent abnormality in children who undergo biopsy because of abnormal urine sediment. However, in a series in which all children with SLE underwent biopsy, only 20% had DPGN.10 Combined data from several large series showed that 42% of children (108 of 256) had DPGN at the time of initial biopsy, 26% had either mild glomerulitis or no abnormality, 25% had focal glomerulitis, and 6% had membranous glomerulonephritis.
Focal glomerulonephritis and membranous glomerulonephritis are generally benign, but either may progress to DPGN with ultimate renal failure.10 Repeat renal biopsy should be performed in affected patients if renal function continues to deteriorate or if they develop persistent hypocomplementemia. Long-term studies indicate that renal scarring (i.e., chronicity index) is a better predictor of the ultimate outcome than the WHO classification.10 In the absence of scarring, active disease (including glomerular crescents) is not automatically associated with a poor prognosis; however, good outcomes for these children are contingent on aggressive management of their renal disease to prevent the development of scarring (see the section on pharmaceutical therapies in this chapter). Most children with SLE do not develop renal disease beyond the first 2 years after diagnosis,2 but one third of those with significant renal disease lack evidence of renal involvement at presentation.
The sudden onset of renal failure in a child with SLE may result from active nephritis, but alternative explanations must be excluded. Renal vein thrombosis and renal artery thrombosis occur in children with SLE and are more frequent in association with anticardiolipin (aCL) antibodies.11 Drugs and health food supplements that interfere with glomerular filtration or are directly nephrotoxic must also be considered. A mild rise in the blood urea nitrogen (BUN) level usually follows the initiation of acetylsalicylic acid or other nonsteroidal antiinflammatory drugs (NSAIDs) in patients with renal involvement, but some children with SLE are unusually sensitive to their effects. An unexpectedly sharp rise in the BUN level after the initiation of NSAIDs should prompt further investigation for renal involvement.
Mild clinical manifestations of renal involvement are usually well controlled with corticosteroid and diuretic agents. Persistent renal disease typically requires immunosuppressive therapy. Chronic glomerular scarring is prevented by cyclophosphamide over the intermediate term.12 The major concern of the physician caring for a child with lupus nephritis is preserving sufficient renal function to support normal growth and development. For female adolescents, this includes the preservation of adequate renal function to support pregnancy. These concerns dictate intervention before significant renal compromise has occurred. Physicians who normally care for adults must be reminded that the normal serum creatinine level of children is much lower. Levels of serum creatinine elevation that might represent minimal impairment in an adult may indicate severe renal compromise in a child.
Current treatment regimens for children and adolescents with lupus nephritis have led to a steady improvement in the survival of 5- and 10-year renal function. However, whether these improvements will result in significantly enhanced survival 20 and 30 years after diagnosis is not yet clear. Maintaining adequate renal function is important for children and adolescents with SLE. In contrast to adults, they progress poorly on long-term dialysis. Children with SLE coming to dialysis with active disease often die of sepsis or other complications within the first year. However, those who have gradually developed global glomerular sclerosis often progress well with dialysis and subsequent renal transplantation.61
Optimal therapy for children and adolescents with lupus nephritis remains uncertain. In large part, this uncertainty is the result of the failure of many investigators to stratify the patients properly in their studies. The systematic use of intermittent intravenous cyclophosphamide has been successful in children with DPGN and useful for children with membranous glomerulonephritis.12,13 More recently, mycophenolate mofetil has shown early promise, but its efficacy in routine use is limited by poor compliance. When the 10-year renal survival is considered, the systematic use of intravenous cyclophosphamide appears to offer the best outcome.13 Newer regimens, which intend to minimize the amount of cyclophosphamide by combining it with rituximab, have shown excellent promise in the short term but have not yet completed long-term follow-up study.14–16
At present, a large-scale study of criteria for evaluating the response to therapy of children with SLE is under way.17 The use of these criteria should improve the ability to assess the various therapeutic regimens that have been advocated for children with lupus nephritis. Routine use of intravenous cyclophosphamide has many advantages, including accurate assessment of patient compliance and clinical status at each dosage interval. Poor compliance is a major determinant of poor outcome. In addition, periodic inpatient cyclophosphamide therapy allows the physician to monitor renal function status and clinical status before each immunosuppressive drug dose, thus minimizing complications. New regimens in which the frequency and total dose of cyclophosphamide are substantially reduced are under investigation. Although consistent use of high-dose mycophenolate mofetil has been recommended for the control of lupus nephritis in adults,18 its sustained benefit is unproven in children and adolescents. Rituximab is a new biologic agent directed against activated B cells bearing the lymphocyte marker CD20. Several case reports regarding its use in SLE and several small series have been published.16,19,20 Additional agents that block or eliminate activated B cells (such as BLyS antagonists) are under evaluation. Regimens using a combination of conventional agents and the newer biologic agents may hold the greatest promise.
Autologous stem-cell transplantation has been proposed and used for a variety of autoimmune diseases including SLE in some children.21 Although this technique may hold great promise, it is associated with a significant mortality and the majority of the reported responses have not persisted over time. Whether the beneficial effect is the result of the stem cell transplantation or of the immunosuppressive chemotherapy given at the time of stem cell transplant is under active investigation.
Central Nervous System Manifestations
Psychosis, sudden personality change, seizures, chorea, transverse myelitis, peripheral neuropathy, and pseudotumor cerebri all may be presenting manifestations of SLE in childhood.3, 22–24 Most series have reported CNS involvement in 20% to 30% of children. If carefully sought, mild evidence of CNS involvement is present in up to 45% of children and adolescents. In every instance, appropriate investigation should be undertaken to exclude stroke as the cause of sudden CNS changes, even in the patient who is not known to be aCL antibody–positive.
Delirium, hallucinations, seizures, and coma are the most common objective neurologic signs in childhood. Psychosis that is unrelated to corticosteroid therapy typically occurs in 4% to 10% of children. The reported frequency of neuropsychiatric manifestations in children and adolescents with SLE is lower than that in adults.25 This finding may be true, but it more likely represents a decreased appreciation of the neuropsychiatric involvement in childhood.
Chorea is more frequent in children than it is in adults with SLE.25 Although infrequent, chorea has been documented as being the initial manifestation of childhood SLE in multiple reports, perhaps because it is such a striking finding. Of children with SLE, 4% to 10% are affected by chorea at some point.22–26 This increased incidence may reflect an increased sensitivity of the basal ganglia to damage by autoreactive antibodies or vascular events accompanying SLE in childhood.
Most often, acute CNS involvement occurs early in the natural history of childhood SLE.25 Frequently, it first becomes evident during or worsens immediately after the initiation of corticosteroid therapy. The explanation for this is uncertain, but these symptoms frequently resolve with pulse methylprednisolone therapy. Late-onset CNS involvement more often is the result of stroke, uremia, or an infectious process.
Both sudden onset of optic neuritis and acute sensorineural hearing loss may occur in children with SLE.27,28 However, the most striking CNS damage in children and adolescents with SLE is typically the result of seizures or strokes, including cerebral vein thrombosis. These complications may occur in the presence or absence of aCL antibodies.22–26
Cognitive defects and aberrant behavior present a more difficult management problem. Aberrant behavior may have dramatic effects on social acceptance, grades, and compliance, thereby directly affecting both self-image and long-term prognosis. Efforts to ascribe behavioral change to a single cause are rarely successful.22–25 Unfortunately, this often results in a failure to aggressively treat these problems with resultant progression.
No single objective test for the presence of CNS involvement in SLE is accurate in childhood. Computed tomography (CT) of children and adolescents with SLE who have received long-term corticosteroid therapy commonly demonstrates diffuse cortical atrophy. Alterations in cerebrospinal fluid protein or sugar levels or cell count are not reliable, but these studies are often necessary to exclude infection and other explanations for altered CNS function.25 Single-photon emission CT may be a more sensitive test for cerebral perfusion abnormalities in these children, but other studies suggest that MRI is more sensitive.23 Antibodies to ribosomal P have been found to correlate with CNS manifestations of SLE in adults, but their presence correlates less reliably with CNS disease in children and adolescents.25
Treatment of CNS manifestations in children and adolescents with SLE is a challenge. Because the manifestations may result from corticosteroid therapy, physicians frequently hesitate to increase the dose; nonetheless, this is often the most effective therapy. For severe CNS manifestations, pulse methylprednisolone therapy is often effective. When other measures fail, intravenous cyclophosphamide is frequently beneficial. Children with short-term psychosis or coma often respond to therapy, but when significant impairment has been present for long periods, the prognosis is guarded. Responding aggressively to continuing evidence of CNS deterioration is important. Chronic, mild problems for which intervention is not believed to be warranted may, nonetheless, progress to dementia over time. In children with active CNS manifestations but relatively normal serum complement levels and only minimal evidence of active SLE in other organ systems, consideration may be given to a trial of anticoagulation instead of increased immunosuppressive agents.29,30
Psychosocial Concerns
Psychological reactions that relate to the many issues affecting children and adolescents with SLE are often confusing. Children with SLE commonly demonstrate an impaired quality of life that is affected by the activity of their disease.31,32 Adolescents who are afflicted with chronic disease are caught between their need to establish an independent personality and the dependency of the sick role. Just as they are struggling to assert their independence, they must be taken for doctor’s visits, are forced to undergo examinations and blood tests, and are required to take unpleasant medications. This situation is intensified by the almost universal need for doses of corticosteroids that increase acne and produce obvious cushingoid facies. The adolescent who does not rebel under these circumstances is unusual. This rebellion may take the form of noncompliance with scheduled physician visits, overt or covert medication noncompliance, or familial disruption. The physician who expects the adolescent with SLE to act like an adult should expect an unsatisfactory patient-physician relationship, which often results in a poor outcome.
Pulmonary Manifestations
Pleurisy and pleural effusions are the most common pulmonary manifestations.33–35 Severe manifestations, including pneumothorax, pneumonia, chronic restrictive lung disease, pulmonary hypertension, and acute pulmonary hemorrhage, may occur.35 Pleuritic chest pain, pleural effusions, and chronic interstitial infiltrates affect 10% to 30% of children with SLE. When a series of Canadian children with SLE were reviewed for manifestations of respiratory involvement, 77% of the patients (17 of 24) had evidence of pulmonary involvement.33
Chronic pulmonary involvement may result in progressive diaphragmatic dysfunction and restrictive lung disease, which result in progressive malaise with dyspnea on exertion and leads to an increased frequency of infection.33,34 Noting both pulse and respiration rates as part of the routine examination is useful. Gradual increases in either or both parameters may be a clue to developing cardiac or pulmonary dysfunction.
A study of 15 children with SLE by Trapani and colleagues36 found pulmonary involvement in 6 patients who were without pulmonary symptoms. Children with dyspnea or tachypnea at rest should be monitored with periodic pulmonary function testing.
Pulmonary hypertension is an ominous finding in children and adolescents with SLE. Once established, it progresses steadily to right ventricular heart failure and death.34 Pulmonary hemorrhage may occur in the setting of preexisting pulmonary hypertension or in isolation.32–36 Sudden unexplained pallor and tachypnea may be the first symptoms of pulmonary hemorrhage,36 which, if left untreated, is rapidly fatal. Children with pulmonary hypertension may benefit symptomatically from the addition of calcium channel–blocking agents or endothelin-1 receptor antagonists to reduce pulmonary vascular resistance. No therapy is known to reverse the course of this complication. Cytotoxic drugs have been ineffective, except in rare anecdotal reports. Pneumonia is a frequent complication in children with established pulmonary hypertension and may progress rapidly to sepsis. Massive pulmonary hemorrhage may respond to large doses of corticosteroids with ventilator support and, perhaps, plasmapheresis or extracorporeal membrane oxygenation.
Minor manifestations of pulmonary involvement normally respond to corticosteroids. Deaths from pneumonia, in which Escherichia coli, the genus Klebsiella, or Staphylococcus aureus were the predominant organisms, illustrate the need for broad-spectrum antibiotic coverage.35 Pneumocystis carinii and other nonbacterial organisms may be present.35 When pneumonia is superimposed on active pulmonary SLE, the contributions of infection and active SLE cannot be differentiated with certainty. Both antibiotics and increased doses of corticosteroids may be appropriate.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree