Chapter 24 Skin Disease in Cutaneous Lupus Erythematosus
Lupus erythematosus (LE) is a multisystem disorder that prominently affects the skin. Cutaneous lesions have a profound effect on quality of life, occur about 50% of the time in the absence of SLE, and can be an indicator of internal disease.1,2
History
The word lupus means “wolf” in Latin, signifying that the destructive injuries caused by the disease were similar to wolf bites. Cazenave coined the term “lupus erythematosus” in 1833 and differentiated between lupus erythematosus and lupus vulgaris, a clinical variant of cutaneous tuberculosis. Owing in part to observations of Hutchinson, it was recognized that cutaneous lesions of lupus erythematosus may be associated with significant systemic disease.3 Starting in 1964, Dubois developed the concept of lupus as a spectrum of disease, ranging from cutaneous disease to life-threatening systemic disease. Gilliam also developed the concept of a spectrum of disease, and, in 1979, described a subset of cutaneous disease, termed “subacute” cutaneous lupus erythematosus (SCLE).4 The description was virtually identical to that of “ANA-negative” lupus reported by Maddison in 1981.5
Hargrave’s description of the LE-cell factor in 19486 and Friou’s subsequent description of the antinuclear antibody assay in 19577 ushered in the era of serologic-clinical correlation in LE. The lupus band test by Burnham, Neblett, and Fine in 19638 and the association of specific autoantibodies, including associations of anti-Ro (also known as anti-SSA anti–Sjögren syndrome antigen A) autoantibodies with neonatal lupus by Franco in 19819 and with subacute cutaneous lupus by Sontheimer in 1982,10 are noteworthy.
In 1951, the synthetic antimalarial quinacrine11 and corticosteroids12 were introduced for the treatment of LE.
Epidemiology
Patients with cutaneous lupus have a lower female-to-male ratio, around 3 : 1, than that seen in patients with SLE. One study found the incidence of CLE to be 4.3 per 100,000 in a predominantly Caucasian population, with a prevalence of 73.24 per 100,000, close to that found for SLE. Twelve percent of the patients with CLE progressed to having SLE, and the average time to progression was 8.2 years.2 Of the various forms of chronic cutaneous lupus erythematosus, discoid lupus erythematosus (DLE) is the most common. Several studies have shown an incidence of 0.6 per 100,000 for SCLE, with lupus panniculitis and bullous LE present at much lower rates, 0.03 to 0.06 per 100,000.2,13 DLE skin lesions are present in 15% to 30% of variously selected study populations with SLE.14 Approximately 5% to 10% of SLE populations have DLE skin lesions as the presenting disease manifestation.15 Patients with SCLE are frequently Caucasian and more predominantly female.13,16
The most common age at onset of DLE is between 20 and 40 years in both males and females.17 DLE lesions, however, can appear in infants as well as the elderly. Patients with SCLE tend to be a bit older, with a mean age of around 60 years.
Triggers of CLE
There are a number of genetic, environmental, and drug-related triggers of cutaneous lupus. Partial C2 and C4 complement deficiencies have been reported in SCLE and chronic cutaneous LE, including DLE and LE panniculitis. Genetic association studies have identified numerous gene polymorphisms that increase the risk for CLE, including genes related to proinflammatory cytokines, tyrosine kinase 2, Fc receptor II (FcRII) and T-cell receptor loci, adhesion molecules, antioxidant enzymes, and apoptosis genes, as well as mutation in TREX1 in familial chilblain lupus.18 The genetics of SCLE are distinct, given the strong association with the HLA-DR3 extended haplotype.19 Ultraviolet (UV) light and visible light can be strong triggers of CLE, and lesions can be induced after natural or experimentally applied light.20
The potential role of medication should be considered in all cases of SCLE, but other forms of CLE are much less frequently due to drugs.21 A number of drugs have been implicated in inducing SCLE (Table 24-1). Ro/SSA autoantibodies have been found in many reported cases of drug-induced SCLE.21 The skin lesions begin as early as 3 days and as late as 11 years, with a median of 6 weeks, after the medication is started, and the lesions typically improve 6 to 12 weeks after the offending agent is withdrawn.21 Smoking raises the risk of CLE, especially DLE.22
TABLE 24-1 Drugs That May Precipitate or Exacerbate LE-Specific Skin Disease
| SCLE | Acebutolol, angiotensin-converting enzyme inhibitors (captopril, cilazapril), antihistamines (cinnarizine, ranitidine, thiethylperazine), calcium channel blockers* (diltiazem, nifedipine, nitrendipine, verapamil), carbamazepine, griseofulvin, hydrochlorothiazide,* interferon-alpha and -beta, leflunomide, naproxen, oxprenolol, D-penicillamine, phenytoin, piroxicam, procainamide, proton pump inhibitors (lansoprazole, omeprazole), spironolactone, statins (pravastatin, simvastatin), sulfonylureas (glyburide), tamoxifen, Taxotere (docetaxel injection), terbinafine,* tiotropium, tumor necrosis factor blockers |
| DLE | Etanercept, infliximab, uracil-tegafur, voriconazole |
| Chilblain LE | Tumor necrosis factor blockers, terbinafine |
| Lupus tumidus | Angiotensin-converting enzyme inhibitors, bupropion, antiretroviral therapy, hydrochlorothiazide |
Clinical Features
Classification of Cutaneous LE
Gilliam developed a classification for cutaneous lupus based on the clinical characteristics of the skin lesions.23 This is the most commonly used classification system at the current time. The skin lesions are separated into lupus-specific and lupus-nonspecific cutaneous lesions (see Boxes 24-1 and 24-2). The lupus-specific lesions are pathognomonic of cutaneous LE, whereas lupus-nonspecific lesions are seen with increased frequency in cutaneous LE but are not always associated with lupus. Lupus-specific skin lesions are separated into acute cutaneous, subacute cutaneous, and chronic cutaneous LE (see Box 24-1). Interestingly, lupus-nonspecific skin lesions are more frequently associated with SLE,24 whereas the presence of lupus-specific skin lesions is relatively protective against severe SLE. Neonatal lupus and bullous lupus are additional cutaneous variants that can be seen in LE.
Box 24-1
Classification of LE-Specific Skin Disease
I. Chronic cutaneous lupus erythematosus (CCLE)
II. Subacute cutaneous lupus erythematosus (SCLE)
III. Acute cutaneous lupus erythematosus (ACLE)
Modified from the Gilliam classification scheme.4
Box 24-2
Classification of LE-Nonspecific Skin Disease
I. LE-nonspecific cutaneous lesions that serve (or served) as classification criteria for SLE
II. LE-nonspecific cutaneous vascular reactions
Modified from the Gilliam classification scheme.4
Lupus-Specific Skin Lesions
Acute Cutaneous LE
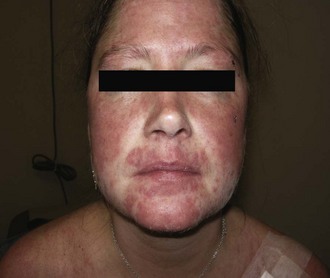
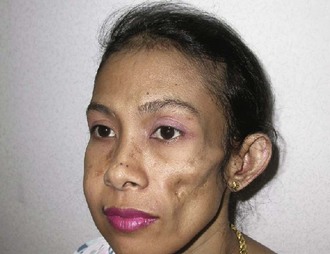
The typical lesion of acute cutaneous lupus (ACLE) is the bilateral malar erythema (“butterfly rash”; Figure 24-1). The lesions tend to be transient, to follow sun exposure, and to resolve occasionally with dyspigmentation but without scarring. Patients presenting with this type of eruption must be evaluated carefully for evidence of internal disease.
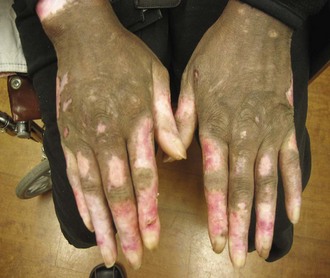
The morphology of the lesions ranges from mild erythema to intense edema. The presence of telangiectasias, dyspigmentation, and epidermal atrophy (i.e., poikiloderma) may help distinguish the malar erythema of acute cutaneous lupus from that of common facial eruptions such as seborrheic dermatitis and the vascular type of rosacea. Occasionally, there is a papular component, and sometimes lesions develop scale or crust. The duration may range from a few hours to several weeks. The face, particularly the malar area, is most commonly affected, with sparing of the nasolabial fold area. Lesions may be more widespread in distribution, with involvement of widespread morbilliform eruption, often in a photoexposed distribution (Figure 24-2). When lesions occur on the hands, the knuckles are typically spared. It is not unusual for the acute cutaneous eruption to be accompanied by oral ulcerations.
Rarely, patients with lupus experience an acute eruption clinically similar to that of toxic epidermal necrolysis (TEN) or erythema multiforme major (Figure 24-3). A TEN-like presentation can occur in patients with LE from extensive interface dermatitis causing epidermal basal cell layer damage. Widespread sloughing of the skin and mucous membranes and full-thickness epidermal necrosis are visualized in biopsy specimens. The presence in patients with lupus of erythema multiforme–like lesions has been termed Rowell syndrome.25 These lesions may represent a severe variant of acute cutaneous lupus or, in some cases, subacute cutaneous lupus.
Subacute Cutaneous LE
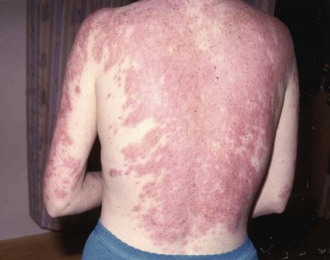
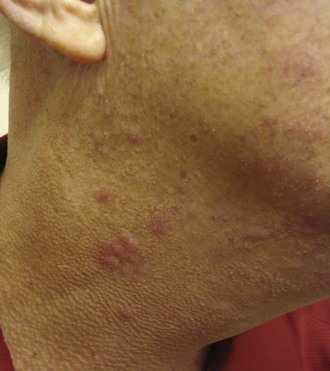
SCLE, defined by Gilliam in 1977, is a distinct subset of cutaneous LE, having characteristic clinical, serologic, and genetic features.23 SCLE is typically photosensitive, although the midfacial skin is usually spared while the sides of the face, V of the neck, and extensor aspects of the upper extremities are commonly involved (Figure 24-4).26 In some patients, the disease may be mild, with only a few small scaly patches appearing after sun exposure.
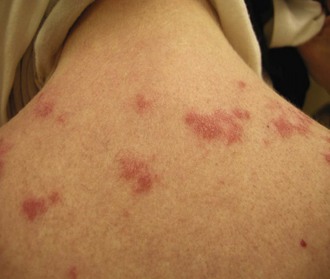
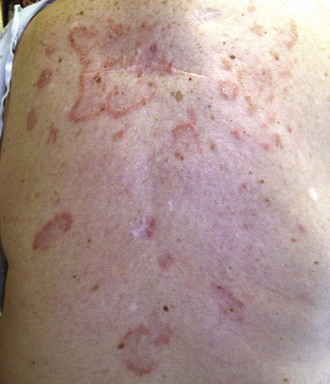
Lesions of SCLE may have an annular configuration, with raised red borders and central clearing (Figure 24-5), or a papulosquamous presentation, with an eczematous or psoriasiform appearance. Both types of lesions can be present in the same patient, although papulosquamous lesions are more common overall. SCLE lesions characteristically have a relatively sparse, superficial inflammatory infiltrate, and consequently, there is usually no induration. Lesions often resolve with dyspigmentation but do not scar. Patients can rarely get blisters in association with SCLE.
In some instances, lesions of SCLE are associated with use of certain medications (see Table 24-1). Drug-induced SCLE is clinically indistinguishable from other forms of SCLE. About one third of these patients have associated antihistone antibodies.21 The lesions normally clear once the medication is discontinued.
Chronic Cutaneous LE
Discoid LE. The most common form of chronic cutaneous LE is classic DLE. Discoid lesions are found most often on the face, scalp, and ears, and lesions above the neck are termed “localized DLE.” The scalp is involved in 60% of patients with DLE and is the only area involved in about 10%. Lesions present both above and below the neck are called “generalized DLE” (Figure 24-6). Patients with generalized DLE have a higher likelihood of meeting criteria for SLE. DLE lesions begin as flat or slightly elevated, well-demarcated, red-purple macules or papules with a scaly surface. Early DLE lesions most commonly evolve into larger, coin-shaped (i.e., “discoid”) erythematous plaques covered by a prominent, adherent scale that extends into dilated hair follicles (Figure 24-7). Involvement of hair follicles is a prominent clinical feature of DLE lesions. Scales accumulate in dilated follicular openings, which soon become devoid of hair. When the adherent scale is peeled back from more advanced lesions, follicle-sized keratotic spikes similar in appearance to carpet tacks can be seen to project from the undersurface of the scale (i.e., the carpet-tack sign). These discoid plaques can enlarge and merge to form even larger, confluent, disfiguring plaques.
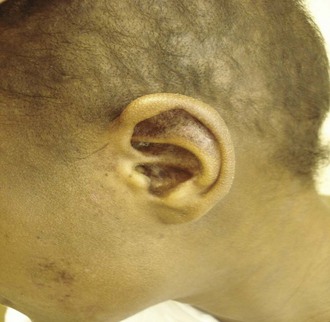
FIGURE 24-7 Scalp and ear involvement with discoid lupus. Note follicular plugging in the ear and scalp.
Hypertrophic/Verrucous DLE.
An unusual variant of DLE is hypertrophic DLE, characterized by thick scaling overlying the discoid lesion or occurring at its periphery. The intensely hyperkeratotic lesions are often prominent on the extensor surfaces of the arms, but the face and upper trunk may also be involved (Figure 24-8). Frequently, typical discoid lesions are also present in other locations. Hypertrophic DLE lesions can easily be mistaken for keratoacanthoma, squamous cell carcinoma, prurigo nodularis, or hypertrophic lichen planus. Thus, a skin biopsy is important to establish the diagnosis.
Lupus Panniculitis/Lupus Profundus.
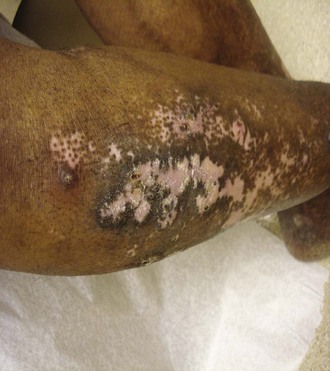
Intense inflammation in the fat leads to indurated plaques that can evolve into disfiguring, depressed areas. Lesions of lupus panniculitis have a distinctive distribution, occurring predominantly on the face, upper arms (Figure 24-9), upper trunk, breasts, buttocks, and thighs. Some patients have discoid lesions overlying the panniculitis, and, in those cases, the condition is sometimes referred to as lupus profundus.
The differential diagnosis of patients with lupus panniculitis includes Weber-Christian panniculitis, factitial panniculitis, pentazocine-induced panniculitis, pancreatic panniculitis, traumatic panniculitis, morphea profundus, eosinophilic fascitis, sarcoidosis, subcutaneous granuloma annulare, subcutaneous T-cell lymphoma, and rheumatoid nodules. Deep excisional biopsy often is required to distinguish LE panniculitis from these other disorders, particularly when overlying classic DLE lesions are not present. The most useful histologic criteria for differentiating LE panniculitis from subcutaneous panniculitis–like T-cell lymphoma are the presence of epidermal involvement, lymphoid follicles with reactive germinal centers, clusters of B lymphocytes, mixed cell infiltrate with plasma cells and polyclonal T-cell receptor γ gene rearrangement. It is helpful to have biopsy specimens reviewed by dermatopathologists, because diagnosis can be difficult, and lupus panniculitis can rarely progress to panniculitic T-cell lymphoma.27
Mucosal DLE.
Mucosal discoid lesions can occur in the mouth most frequently but can also involve the conjunctiva, nose, and genitals. The prevalence of mucous membrane involvement in chronic, cutaneous LE is about 25%. Within the mouth, the buccal mucosa is most commonly involved, and the palate, alveolar processes, and tongue are less frequently involved. The center of an older lesion can become depressed and occasionally undergoes painful ulceration. Well-defined chronic DLE plaques also can appear on the vermilion border of the lips. At times, DLE involvement of the lips can manifest as a diffuse cheilitis, especially on the more sun-exposed lower lip. Although lesions can appear on the tongue, this location is quite uncommon. Chronic oral-mucosal DLE lesions occasionally can degenerate into squamous cell carcinoma, like cutaneous DLE lesions. Any area of asymmetric nodular induration within a mucosal DLE lesion should be carefully evaluated for the possibility of malignant degeneration. Conjunctival DLE lesions begin as small areas of nondescript inflammation most commonly affecting the palpebral conjunctivae or the margin of the eyelid. The lower lid is affected more often than the upper lid. As the early lesions progress, scarring becomes more evident and can produce permanent loss of eyelashes and ectropion. DLE involvement of the eyelid can produce considerable disability. Lid deformities trichiasis, and symblepharon formation can also occur as a result of DLE ocular involvement.81
LE Tumidus/Papulomucinous LE.
Some patients with cutaneous lupus have lesions characterized by induration and erythema but no scale or follicular plugging. Lesions can be common on the face and trunk and can be edematous (Figure 24-10). Morphologically, the lesions are similar, if not identical, to those of Jessner lymphocytic infiltrate and may have central clearing. The epidermis typically is uninvolved in the disease process, lacks the liquefactive degeneration and basement membrane thickening typically seen in DLE and SCLE, but has an intense dermal inflammatory infiltrate.28 These lesions are called LE tumidus, or tumid LE.
The very low prevalence of SLE and the relatively low prevalence of immunoglobulin deposition within the cutaneous lesions in patients reported to have tumid lupus have made it difficult to determine whether tumid lupus is actually a variant of lupus erythematosus or an independent entity. The presence of tumid lupus lesions in patients with other specific types of cutaneous lupus is evidence in favor of its being classified as a form of cutaneous lupus. Tumid lupus has been reported to be reproducible by phototesting in the majority of patients.29 The lesions tend to resolve without scarring or atrophy.
Chilblain LE
Lichen Planus–Lupus Erythematosus Overlap.
Overlap between lupus erythematosus and lichen planus has been observed in a small number of patients. This overlap syndrome is characterized by the presence of clinical, histologic, and/or immunopathologic features of both diseases in the same patient. Such patients have had mainly painful, bluish red plaques with atrophy and scaling as well as hyperkeratotic papules and nodules that favor extremities.30 Pathologic differences can help differentiate the two entities, with colloid bodies in the dermis and basement membrane clefts seen in lichen planus, and basement zone thickening observed in lupus. Patients with this overlap syndrome may have an autoimmune, viral, and/or genetic predisposition. Successful treatments have included acitretin and cyclosporine.30
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree