Chapter 37 Neonatal Lupus Erythematosus
The association of neonatal cardiac and skin disease with maternal systemic lupus erythematosus (SLE) was first identified through case reports in the 1950s and 1960s.1,2 Since then, several clinical manifestations, most importantly congenital heart block (CHB), but also neonatal skin lesions, transient hematologic and a liver abnormalities, central nervous system (CNS) involvement, and rare bone disease, have all been linked to in utero exposure to maternal anti–Sjögren syndrome antigen A (anti-SSA/Ro) or anti–Sjögren syndrome antigen B (anti-SSB/La) antibodies3 (Table 37-1).
TABLE 37-1 Frequency of Different Organ Manifestations in Children Born to Mothers Who Are Positive for Anti–Sjögren Syndrome Antigen A
| ORGAN AND TYPE OF MANIFESTATION | REPORTED FREQUENCY | REFERENCE |
|---|---|---|
| Heart | ||
| First-degree atrioventricular block1 | 10%-14% | 70, 100 |
| Third-degree atrioventricular block | 2% | 10 |
| Skin | 25% | |
| Liver | 9%-26% | 15, 16 |
| Elevated transaminase enzymes | 25% | 15 |
| Hematologic manifestations | 27%-50% | 15 |
| Neutropenia | 23% | 15 |
| Anemia | 5% | 15 |
| Thrombocytopenia | 4% | 15 |
| Nervous system | ||
| Hydrocephalus (transient) | 10% | 17 |
| Nonspecific white matter changes | 8% | |
| Bone | ||
| Chondrodysplasia punctata | Rare | 91, 92 |
1 Observed by postnatal electrocardiographic examination.
Maternal IgG antibodies of all subclasses are transported across the placenta, starting at approximately 16 weeks’ gestation. Although the complete spectrum of maternal IgG specificities, including autoantibodies, cross the placenta, the vast majority of cases of neonatal lupus erythematosus (NLE) are associated with anti-SSA/Ro and anti-SSB/La antibodies, with a few cases associated with antiribonucleoprotein (anti-RNP) or antihistone antibodies.4–6 Infants born to women with these antibodies are expected to have circulating maternal autoantibodies at decreasing levels for the first 3 to 6 months of life.7,8
The majority of infants born to women with anti-SSA/Ro antibodies are born without obvious abnormalities or illnesses. Cutaneous disease occurs in up to 25% of infants exposed to anti-SSA/Ro antibodies, but it is mild and resolves without diagnosis or significant acknowledgment in many patients.9 Complete CHB occurs in up to 2% of infants exposed to SSA/Ro antibodies, with a recurrence rate between 12% and 20%.10–13 The occurrence of CHB in infants born to women with a prior infant with NLE involving the skin has been reported as 13%.14 Hematologic manifestations often go unnoticed in an otherwise healthy infant but can be found in up to 50% of tested infants.15 Mild elevations of transaminase enzymes typically remain asymptomatic but can be identified in 25% of tested infants.15,16 Neurologic abnormalities, including hydrocephalus and nonspecific white matter changes, which are visualized with brain computed tomography (CT), have been reported in fewer than 10% of exposed infants, often without symptoms.17,18 A rare skeletal disorder, chondrodysplasia punctata, may also be associated with in utero exposure to maternal autoantibodies.3,19
Maternal disease manifestations before and during pregnancy do not appear to have a significant impact on neonatal outcomes. The mothers may be diagnosed with Sjögren syndrome (SS) or SLE; however, fewer than 20% of the women fulfill the criteria for a rheumatic disease at the time that CHB is detected in the fetus, although many mothers display symptoms of an undifferentiated connective tissue disease and have complaints such as dry eyes, dry mouth, fatigue, or photosensitivity.20,21 Approximately one half of the women without a diagnosis will progress to rheumatologic disease over the subsequent 3 to 6 years, most commonly SS or SLE.21,22
Etiologic Factors and Pathogenesis
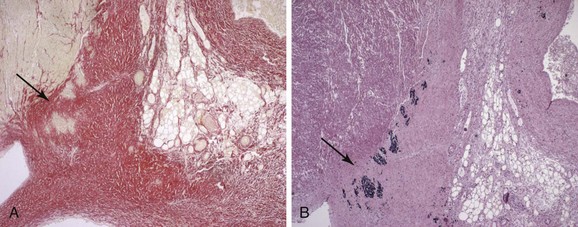
The close association of NLE with maternal SSA/Ro and SSB/La antibodies rather than manifest clinical rheumatic disease led to the hypothesis that the antibodies have a direct role in disease pathogenesis. The histopathologic examination of the hearts of fetuses who died of CHB support antibody involvement and an inflammatory reaction as part of the process leading to conduction failure, with the presence of Ro-specific immunoglobulin and complement deposits, inflammatory cells dominated by macrophages, and cytokine expression, including tumor necrosis factor–alpha (TNF-α) and transforming growth factor–beta (TGF-β).23–25 Calcification and fibrosis denote end-stage destruction of the atrioventricular (AV) node and will clinically correspond to complete, third-degree AV block (AVB) (Figure 37-1). Of note, antibodies, complement deposits, and signs of fibrosis and calcification can be observed not only at the AV node but also in the entire myocardium, suggesting a potential involvement of maternal autoantibodies in other cardiac manifestations of CHB, such as sinus bradycardia and cardiomyopathy.
Maternal Autoantibodies in Congenital Heart Block
The association between maternal SSA/Ro and SSB/La autoantibodies and CHB was described in the early 1980s.26,27 The observation that the SSA/Ro autoantigen consists of two unrelated proteins, Ro52 and Ro60,28,29 and subsequent studies of the CHB association with maternal antibodies have led to efforts determine the serum profile of mothers of affected children regarding the three components, Ro52, Ro60, and La. Although the data vary among the different studies, depending on the methods used for antibody detection, the enrollment criteria for pregnancies, as well as the definition of CHB, most of the attempts demonstrate that anti-Ro and especially anti-Ro52 antibodies are present in a high proportion of mothers of children with CHB.4–6,30–32 The close correlation between maternal anti-Ro52 antibodies and CHB in combination with the fact that only 1% to 2% of children born to women who are anti-Ro positive develop heart block, has prompted a search for a specific profile within the pool of maternal anti-Ro52 antibodies. Dominant epitopes within the central part of the Ro52 protein have been described in the context of SLE and SS,33,34 and epitope mapping using overlapping peptides covering this region revealed a significant association between maternal antibodies to amino acids 200-239 of Ro52 (denoted p200) and the risk for CHB.6,30,35 In a prospective study of women who were anti-Ro52 positive during weeks 18 through 24 of pregnancy, maternal antibodies to Ro52/p200 were shown to correlate to longer AV time intervals in the fetuses.36
As anti-Ro60 and anti-La antibodies are most often found with anti-Ro52 antibodies, assessing their individual contribution to the development of CHB is difficult. In addition, most studies still rely on clinical assays that do not distinguish between Ro52 and Ro60 to investigate the presence of anti-Ro antibodies in maternal sera. In two studies, the levels of anti-La antibodies were found to be higher in mothers of children with cutaneous NLE than in women giving birth to a child with CHB.37,38 However, another study suggested that the risk for CHB was increased in the presence of anti-La antibodies.39 The current consensus is that antibodies to Ro60 and La may contribute to the inflammatory reaction that leads to AV block but CHB may develop in their absence.
Considering the low risk for fetal heart block in an anti-Ro–positive pregnancy (2%), a search for other antibodies associated with heart block has been undertaken by different research groups and has yielded some candidates. However, this small number of studies has often involved too few infants to demonstrate a reliable association between the presence of antibodies and pregnancy outcomes. Thus antibodies to calreticulin, a protein involved in calcium storage, have been found more frequently in sera from mothers of children with CHB than in sera from mothers of healthy children.40 Antibodies recognizing the muscarinic acetylcholine receptor M1 have also been associated with the development of CHB, and in vitro studies suggest a functional role for these antibodies through binding to and interfering with the function of their target in the neonatal myocardium.41,42 In addition, antibodies recognizing a cleavage product of α-fodrin have been proposed as an additional serologic marker for heart block.43 Similarly, reactivity to the α1D calcium channel subunit was recently found in sera from mothers of children with CHB; however, such reactivity was limited to approximately 14% of all mothers of infants with CHB who were tested.44
To date, anti-Ro52 antibodies seem to remain the maternal autoantibodies that correlate to the development of CHB to the greatest extent, despite the low penetrance of the condition in anti-Ro–positive pregnancies. It is possible that not only the presence but also the levels of maternal anti-Ro52 antibodies are of importance in predicting fetal outcome, as is suggested in a recent study in which cardiac conduction disturbances were associated with moderate to high levels of anti-Ro antibodies but not with low levels.38
Clues to Pathogenic Mechanisms in Congenital Heart Block from Experimental Models
Direct evidence of a pathogenic role of maternal anti-Ro and anti-La antibodies in CHB come from experimental in vitro and in vivo studies of heart block. In vitro studies on rat or human hearts perfused with the Langendorff technique have demonstrated a direct pathogenic role of antibodies from mothers of children with CHB, because maternal IgG containing anti-Ro or anti-La antibodies induced bradycardia and complete AV block within 15 minutes.45,46 Affinity-purified anti-Ro52 antibodies had the same effects, showing the individual pathogenic potential of anti-Ro52 antibodies. Similar results were obtained in Langendorff-perfused rabbit hearts exposed to anti-Ro or anti-La antibodies purified from mothers of children with CHB.47,48
Evidence for the pathogenicity of anti-Ro or anti-La antibodies in vivo has been gathered from animal models based on the passive transfer of antibodies or active immunization of women before gestation. Transfer of affinity-purified anti-Ro or anti-La antibodies from mothers of children with CHB into pregnant female BALB/c mice induced first-degree AV block and sinus bradycardia in the offspring.49 Immunization models of CHB, in which female rats, mice, or rabbits were injected with a particular antigen before gestation, made it possible to investigate separately the pathogenic potential of antibodies toward Ro52, Ro60, or La. Immunization of BALB/c mice with Ro60 or La led to the development of first-degree AV block in 19% or 7% of the offspring, respectively,50 and similar results were observed in C3H/HEJ mice.51 Immunization of mice, rats, or rabbits with the human or mouse Ro52 protein induced first-degree AV block in 9% to 45% of the offspring45,50,52,53 but also higher degrees of AV block and rates of neonatal deaths.45,50,52 The AV block–inducing capacity of Ro52 antibodies and the fine specificity of the Ro52 antibodies inducing block have been further confirmed by both immunization with the Ro52-p200 peptide36 and the passive transfer of monoclonal antibodies targeting different epitopes in different domains of the Ro52 protein.54 In the transfer of Ro52 monoclonal antibodies to pregnant rats, only antibodies targeting amino acids 200-239 of Ro52 induced AV block, which was observed in 100% of exposed pups.54
Targets for Maternal Antibodies in the Fetal Heart
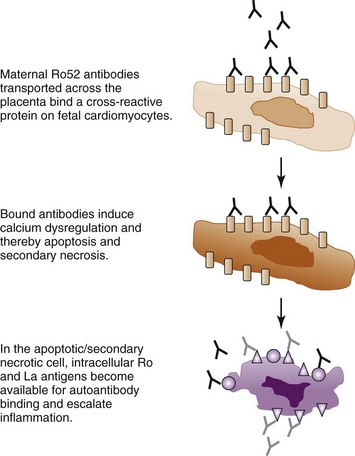
The apoptosis hypothesis postulates that maternal antibodies gain access to their target antigen when it is exposed on the surface of apoptotic cells. The presence of Ro60 or La has indeed been reported on apoptotic cardiac myocytes.50 Ro52 has also been detected on the surface of apoptotic but not live cardiac cells in one study, although only one out of the five anti-Ro52 monoclonal antibodies tested bound apoptotic cells and did so to a lesser extent than did anti-Ro60 or anti-La antibodies.55
The apoptosis hypothesis fails, however, to explain the rapid electrophysiologic effects of maternal anti-Ro or anti-La antibodies on Langendorff-perfused hearts and the specificity of the reaction in targeting the AV node. The cross-reactivity hypothesis therefore suggests that maternal anti-Ro and anti-La antibodies, or at least a subset of these, bind to cardiac membrane proteins involved in the control of electric signal generation or conduction or both, interfering with their function. The involvement of maternal anti-Ro52 antibodies cross-reacting with the serotoninergic 5-hydroxytryptamine (5-HT4) receptor was suggested after Eftekhari and colleagues56 found that antibodies to the Ro52 peptide 365-382 recognized residues 165-185 of the cardiac 5-HT4 receptor and that affinity-purified 5-HT4 antibodies could antagonize the serotonin-induced calcium channel activation in atrial cells.57 However, only 16% of the sera from mothers of children with CHB were shown to be positive for anti–5-HT4 antibodies, indicating that cross-reactivity to the serotoninergic 5-HT4 receptor, if indeed involved in the development of CHB, may only represent a small subset of cases.58
Calcium channels constitute another group of molecules investigated for an involvement in CHB. IgG purified from mothers of children with CHB inhibits L-type and T-type calcium currents in ventricular myocytes, as well as in sinoatrial node cells and exogenous expression systems.46,52,59–61 Experimental data supporting a possible cross-reactivity of maternal anti-Ro or anti-La antibodies with the α1C and α1D calcium channel subunits have also been provided.60,61 Further, mouse pups transgenic for the L-type calcium channel, voltage-dependent, α1C subunit (Cav 1.2) were found to develop AV block and sinus bradycardia at a lower frequency than nontransgenic littermates after in utero exposure to anti-Ro or anti-La antibodies in an immunization model.44 In addition, mouse pups in which the Cav1.3 subunit of the L-type calcium channel has been genetically knocked out exhibit first-degree AV block, and the occurrence of AV block is increased after immunization of the female mice with the Ro and La protein before gestation.62 A specific effect of Ro52 antibodies targeting the p200 epitope was demonstrated as p200-specific monoclonal antibodies that induced AV block in vivo also dysregulated calcium oscillations of spontaneously beating primary neonatal cardiomyocytes in culture.54 Although these studies do not prove that maternal anti-Ro and anti-La antibodies directly cross-react with subunits of the L-type calcium channel, they support the hypothesis that maternal autoantibodies exert their pathogenic effects at least in part by affecting calcium homeostasis in the heart and disrupting the cardiac electric and contractile functions. Prolonged disruption of cardiac calcium homeostasis may possibly lead to increased apoptosis in the fetal heart,36 which would then be accompanied by exposure of the intracellular Ro and La proteins and allow for the establishment and amplification of an inflammatory reaction as described in the apoptosis hypothesis, leading to irreversible damage and complete CHB (Figure 37-2).
Additional Risk Factors in Congenital Heart Block Development
A risk of 2% for CHB in an anti-Ro–positive pregnancy and a reported recurrence rate of only 12% to 20%,10–13 despite persisting maternal antibodies, indicate that additional factors are critical for the establishment of heart block. Epidemiologic, environmental, and genetic factors have been investigated in this respect (Table 37-2).
TABLE 37-2 Factors Examined and Influencing or Not Influencing the Risk for Congenital Heart Block
| PARAMETER | INFLUENCES | REFERENCE |
|---|---|---|
| Maternal Ro/La antibodies | Yes | 5, 6, 26, 27, 112 |
| Maternal age1 | Yes | 8, 13 |
| Previous congenital heart block (CHB) pregnancy2 | Yes | 11–13 |
| Increasing parity | No | 13 |
| Maternal disease activity | No | 32, 63 |
| Fetal gender | No | 13 |
| Season of birth3 | Yes | 13 |
| Maternal histocompatibility complex (MHC)4 | Yes | 53, 113 |
| Fetal MHC5 | Yes | 53, 65, 114 |
1 The odds ratio for CHB increases by four in women 35 years of age and older, compared with women 24 years of age or younger.
2 The risk for CHB increases six- to ten-fold in pregnancies after a CHB pregnancy in mothers who are positive for Ro/La antibodies.
3 The risk for CHB increases in gestational weeks 18 to 24.
4 Maternal human leukocyte antigen (HLA)–DRB1*03 is more frequently observed in mothers of children with CHB than in the general population.
5 Fetal MHC genes influence the risk for the development of CHB, with the tumor necrosis factor–alpha (TNF-α) polymorphism and HLA-Cw3 identified as genetic factors.
Although neither fetal gender nor maternal disease severity has been associated with CHB,13,32,63 it has been proposed that maternal age or parity or both may have an influence on the outcome of anti-Ro52–positive pregnancies.8 An analysis of risk factors for the development of heart block in a population-based Swedish cohort demonstrated that the risk for CHB increased with maternal age but was not influenced by parity.13 In addition, the seasonal timing of the pregnancy influenced the outcome, with an increased proportion of affected pregnancies when the susceptibility weeks (18 to 24 weeks’ gestation) fell in the late winter season. An association of the winter season with decreased sun exposure and vitamin D levels readily comes to mind; in addition, however, other events linked to the winter season such as viral infections may provide the mechanistic explanation for the seasonal influence on the development of heart block.
Genetic polymorphisms influencing fetal susceptibility to CHB in anti-Ro– and anti-La–positive pregnancies were first investigated in a group of 40 children with CHB using a candidate-gene approach and focusing on two known polymorphisms of the genes encoding the proinflammatory and profibrotic cytokines TNF-α and TGF-β. The TGF-β polymorphism assessed was found significantly more frequently in children with CHB, whereas the TNF-α polymorphism studied was found at an increased frequency in both children with CHB or rash, compared with those in the healthy control group.64 These findings have, however, not yet been replicated in a large group of infants with CHB. More recently, a genome-wide association study of infants with CHB born to anti-Ro– and anti-La–positive mothers was performed and a significant association with polymorphisms in the HLA region and at the location 21q22 reported, compared with those in the healthy control group.65 Although the association with the major histocompatibility complex (MHC) locus is supported by experimental studies in an animal model,53 one should be careful in the interpretation of the genetic associations because the studies presented so far were performed by comparing infants with CHB with healthy control infants from the general population. The associations may therefore reflect the genetic bias present in the mothers who may have SLE or SS or, even if asymptomatic, have autoantibodies to the Ro/La autoantigens.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree