Fig. 26.1
Schematic drawing of the skeletal muscle organization
Most of the skeletal muscles are derived from somatic mesoderm. In the embryo, a muscle fiber is formed by the fusion of a number of myoblasts, which are small, mononucleated, immature muscle cells and derived from pluripotent mesenchymal stem cells [2–5, 7]. Skeletal muscle fibers are long, cylindrical, multinucleated cells, and peripheral location of numerous nuclei just beneath the sarcolemma (cell membrane) is typical (Figs. 26.2 and 26.3). The diameter of a mature muscle fiber varies from 10 to 100 μm. The length of a mature muscle fiber is related with its location and varies from a few millimeters to about 1 m [1–4, 7]. Males have greater muscle mass than females. Size of the individual muscle fibers is larger in males due to bigger body size, more physical exertion, and hypertrophic effect of androgens on muscle fibers [5]. Experimental studies have shown that the length of muscle fibers is directly proportional with the skeletal muscle excursion (maximum contraction velocity). Besides, the diameter of the muscle fibers is directly proportional with the force generated by the skeletal muscle [1].
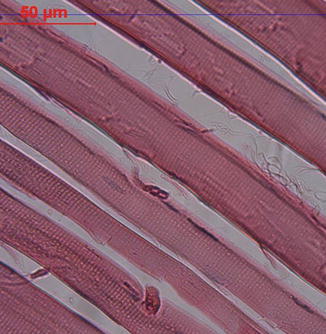
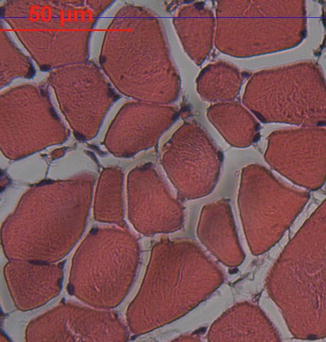

Fig. 26.2
Longitudinal section of a skeletal muscle at the light microscopy level. Cross-striations due to contractile proteins are seen (H.E., ×200)

Fig. 26.3
Transverse section of a skeletal muscle at the light microscopy level. Peripheral localization of numerous nuclei in muscle fibers is seen (H.E, ×200)
Satellite cells are small cells with single nucleus and localized in shallow depressions on muscle fibers’ surfaces. They act like stem cells in the repair process and following a muscle injury, they proliferate to give rise to new myoblasts [2, 3, 5].
The structural and functional subunit of a muscle fiber is myofibril. Most of the muscle fiber cytoplasm (sarcoplasm) is filled with myofibrils, which are cylindrical in shape, arranged as parallel arrays extending the entire length of muscle fibers and having a diameter of 1–3 μm and a length of 1–2 cm [2–4, 8]. Myofibrils are composed of bundles of thick and thin myofilaments. Thick myofilaments, which are 1,5 μm long and 15 nm in diameter, are composed of the protein myosin. Each thick myofilament contains about 200–300 myosin proteins [2–5, 7]. Thin myofilaments, which are 1 μm long and 6–8 nm in diameter, are mainly composed of the protein called the actin and troponin, tropomyosin, as well [2–5, 7]. Each thick myofilament is equidistantly surrounded by six thin myofilaments in a mammalian skeletal muscle [3]. Accessory proteins, including titin, α-actinin, nebulin, tropomodulin, desmin, myomesin, C protein, and dystrophin regulate the spacing, attachment, and alignment of the myofilaments [2]. Myosin and actin constitutes more than half of the proteins found in the striated muscle [4].
Cross-striations of muscle fibers can be visible in unstrained preparations examined in polarizing microscope and look as repetitive light and dark bands. In polarizing microscopy, dark bands are anisotropic or double refractive and named as A bands (Fig. 26.4). A bands contain both thick and thin myofilaments. A band is bisected by a light region called the H band (Fig. 26.4). H band contains only thick myofilaments. H band is bisected by a narrow and dense line called the M line (Fig. 26.4). M line is formed by the linkage of adjacent thick myofilaments [1–4]. Light bands are isotropic and named as I bands (Fig. 26.4). I bands contain only thin myofilaments [1–4]. I band is bisected by a dense line called the Z line (Z disk) (Fig. 26.4). The segment of the myofibril between two adjacent Z lines is called the sarcomere. It is the basic contractile unit of striated muscle and its length is about 2–3 μm in resting mammalian muscle. Its length decreases during muscle contraction and increases in muscle stretching. The length of A band remains unchanged during resting, contraction, or stretching; however, the lengths of H and I bands change according to the status of the skeletal muscle [1–5, 7] (Fig. 26.4).
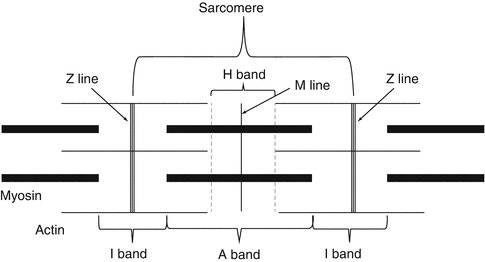

Fig. 26.4
Schematic drawing of a sarcomere
Most of the intracellular organelles of the muscle fiber are located around the nucleus and myofibrils. Sarcoplasmic reticulum is the intracellular organelle, placed around the myofibrils as a meshwork and forms a channel called the terminal sac at the A-I band junction. Terminal sac of sarcoplasmic reticulum is the main store of Ca2+, which is the essential ion for initiating muscle contraction. T tubules are the tubular invaginations of the sarcolemma and located at the A-I band junction. They carry the impulses from sarcolemma to terminal sac. The complex of two neighboring terminal sacs and one T-tubule is called the triad. As the reactions initiating the contraction process need energy, variable numbers of mitochondria, 1 μm in width each, are located around the myofibrils and close to the Z line. Besides, numerous glycogen molecules are located close to myofibrils [1–5, 7].
Types of Muscle Fibers
Skeletal muscle fibers have a wide spectrum of morphologic, contractile, and metabolic properties. Several classification schemes have been defined for typing the muscle fibers [1] (Table 26.1). Fast contracting glycolytic muscle fibers are large in cross section, have the highest number of glycogen molecules and glycolytic enzymes, so are rich in ATP and adapted for sudden and stronger contraction to make the precise and rapid movements available, but fatigue rapidly. Such muscle fibers are mostly found in muscles controlling the movements of digits [1–3, 7]. Slow contracting oxidative muscles are small in cross section, contain abundant mitochondria, therefore are rich in oxidative enzymes and myoglobin, adapted for slow, weaker, repetitive contraction but have more resistance to fatigue. Such muscle fibers are found in limb and long back muscles [2, 3, 5, 7]. While the number of fast glycolytic muscle fibers is higher in sprinters, the number of slow oxidative muscle fibers is higher in long distance runners [5, 8].
Basis for classification | Muscle fiber subgroups | ||
|---|---|---|---|
Metabolic | Slow contracting, oxidative | Fast contracting, oxidative glycolytic | Fast contracting glycolytic |
Morphologic and physiologic | Slow contracting red | Fast contracting white | Fast contracting white |
Z-line width | Wide | Intermediate | Narrow |
Histochemical | Type 1 | Type 2A | Type 2B |
Immunohistochemical | Type 1 | Type 2A and 2X | Type 2B |
Clinical Relevance
Muscle hypertrophy is the increase in the diameter and volume of muscle fibers due to several factors such as nutrition and exercise. Muscle atrophy is decrease in the diameter and volume of muscle fibers due to factors like aging and immobilization [4, 5, 8].
Muscle hyperplasia is the increase in the number of muscle fibers and does not occur in a healthy skeletal muscle [4].
Muscle dystrophies are a group of inherited disorders with progressive degeneration and weakness of skeletal muscles. Duchenne’s and Becker’s muscular atrophies are the most common types and due to absence of dystrophin in myofibrils as a result of mutation in encoding dystrophin [9].
In pes equinovarus due to myelomeningocele, the diameter of muscle fibers decreases except the gastrocnemius muscle and the most severe atrophy and fibrosis is seen in the long peroneal muscle [10].
The use of tourniquets on extremities during surgery can have adverse effects on the ultrastructure of muscle fibers distal to the tourniquet. Early ultrastructural findings of muscle atrophy can be seen in muscles distal to tourniquet if the tourniquet time exceeds 1 h [11]. Besides, post-ischemic reperfusion can be seen following the use of tourniquet. This increases the capillary permeability and leads to oxidative stress [11, 12].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








