Fig. 38.1
Implant failure due to exogenous osteomyelitis after femur fracture treatment with plate
After removal of all implants and proper debridement, a unilateral external fixator was applied to the femur (see Fig. 38.2). Antibiogram showed methicillin-resistant Staphylococcus aureus (MRSA) infection. Patient had teicoplanin plus ciprofloxacin treatment for 6 weeks [3]. Serum levels of ESR and CRP were normalized after regular treatment with antibiotics. After 4 weeks without antibiotic treatment, patient showed no signs of infection. All laboratory findings were normal [4]. Her fracture was fixed with a long plate and the defect in the fracture site was filled with an autolog (tricortical iliac crest) bone graft (see Fig. 38.3). Two months after the procedure, consolidation of the fracture was achieved without any signs of infection. Patient did not have any other complications other than a slightly limited range of motion (ROM) in her knee (0–90), and she was satisfied with the results of definitive treatment.
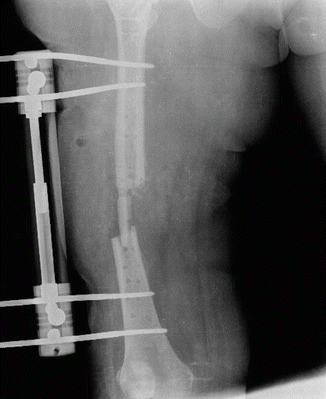
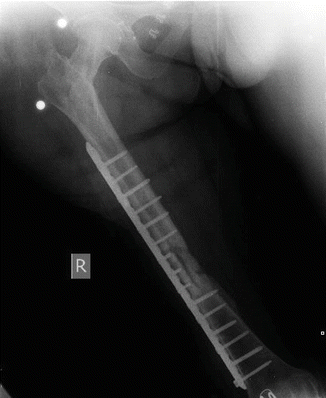

Fig. 38.2
After removal of all implants and proper debridement

Fig. 38.3
Bone gap was filled an autolog bone graft and fixed with a long plate again
The use of antibiotics in orthopedic surgery has become popular since the discovery of penicillin during the Second World War. Penicillin is a group of antibiotics derived from Penicillium fungi. All penicillins are bactericidal, β-lactam class antibiotics and used in the treatment of infections caused by mostly Gram-positive and other susceptible bacteria. They inhibit the formation of peptidoglycan cross-links in the bacterial cell wall. Peptidoglycan, also known as murein, is a polymer that forms a mesh-like layer outside the plasma membrane of all bacteria except the mycoplasma class. The β-lactam ring of penicillin binds and inhibits the trans-peptidase enzyme. Then, the formation of cross-links in the bacterial cell wall is disrupted and an imbalance between cell wall production and degradation develops, causing the bacteria to die. β-lactam antibiotics are structural analogs of D-alanyl-alanine, and the transpeptidase enzyme that binds to them is also called penicillin-binding protein (PBP). In essence, penicillins can show their bactericidal effect on immature and colonizing bacteria that have murein in their cell wall. Penicillin was used frequently from the beginning of the 1940s to the 1970s. However, use of advanced penicillin became popular after increased resistance developed among bacterial pathogens to first-generation penicillin.
Meticillin (or methicillin) is a narrow-spectrum β-lactam antibiotic from the penicillin class. Similar to other β-lactam antibiotics, meticillin disrupts the synthesis of bacterial cell walls by inhibiting cross-linkage between peptidoglycan chains. Meticillin is a penicillinase-resistant antibiotic. Resistant bacteria produce an enzyme called penicillinase, which hydrolyses the β-lactam antibiotic and renders it non-functional. However, this enzyme does not bind to meticillin, meaning it can still function to kill the bacteria. Previously, meticillin was used to treat infections caused by Gram-positive bacteria including Staphylococcus aureus (S. aureus), Staphylococcus epidermidis (S. epidermidis), Streptococcus pyogenes (S. pyogenes), and Streptococcus pneumoniae (S. pneumonia). But today, due to resistance meticillin is not as effective against these microorganisms. A newer PBP gene called mec2 confers resistance to meticillin. This gene produces PBP2a, which works in a similar way to other PBPs though it binds β-lactam with a very low affinity. Expression of PBP2a supplies resistance to all β-lactam antibiotics. Recently, meticillin has been used less frequently than before because it is less active compared to other β-lactamase-resistant penicillin, and can be administered only via a parenteral route and has a higher frequency of interstitial nephritis. It has been largely replaced in its role in therapy by other penicillins such as dicloxacillin and flucloxacillin, but the term meticillin-resistant S. aureus (MRSA) continues to be used to describe S. aureus strains resistant to all penicillin.
The cephalosporins are a class of β-lactam antibiotics and they derive from the fungus Acremonium, known as Cephalosporium. The first-generation cephalosporins were not used for prophylaxis in orthopedic surgery until 1964. First-generation cephalosporins are active principally against Gram-positive bacteria, though their successors have increased activity against Gram-negative bacteria. Cephalosporins are bactericidal, meaning that they kill the targeted bacteria as opposed to inhibiting reproduction as bacteriostatic antibiotics do. They have the same path of action as other β-lactam antibiotics such as the penicillin class. They disrupt the synthesis process of the peptidoglycan layer, which is essential for bacterial cell wall structural integrity. They are resistant to β-lactamase of S. aureus, and this makes them preferable for prophylaxis and treatment. First-generation cephalosporins are narrow-spectrum antibiotics and they are active against Gram-positive (S. aureus, S. pyogenes, and S. pneumonia) and some strains of Gram-negative coccus bacteria. They are also active against a small number of Gram-negative aerobic bacilli such as E. coli, Klebsiella, P. mirabilis, N. gonore, and N. meningitidis. Cefazolin has the longest half-life among first-generation cephalosporins. It can be administered via intravenous (IV) or intramuscular (IM) routes and is tolerated well by the patient. Since it has a long serum half-life, it is recommended for surgery prophylaxis and doses should be given every 8 h. It is relatively well tolerated by the patient as it causes less pain around the injection site.
Vancomycin is from the glycopeptide antibiotic class and is effective mostly against Gram-positive bacteria. Vancomycin shows its bactericidal effect by inhibiting proper cell wall synthesis in Gram-positive bacteria. Vancomycin forms hydrogen bond interactions with the terminal D-alanyl-D-alanine parts of the N-acetylmuramic acid (NAM) and N-acetylglucosamine (NAG) peptides. This is a five-point interaction. This binding disrupts cell wall synthesis in two ways. It prevents the synthesis of the long polymers of NAM and NAG that are essential for bacterial cell wall structure, and it prevents the formation of cross-links between these polymers. Since Gram-negative bacteria produce their cell walls by different mechanisms and there are various factors related to entering the outer membrane, vancomycin is not active against Gram-negative bacteria (except non-Gonococcal species of Neisseria). The activity of vancomycin is considered to be time dependent, which means antimicrobial activity depends on the duration that the serum drug concentration exceeds the minimum inhibitory concentration of the target organism. Vancomycin is a large hydrophilic molecule and must be administered via intravenous route for systemic therapy, since it is not absorbed from the intestine. Due to its short half-life (4–11 h in adults), it is often injected twice daily. Common adverse drug reactions (≥1 % of patients) associated with IV vancomycin include local pain, which may be severe, and thrombophlebitis. IV vancomycin therapy using peripheral lines inherently causes risk of thrombophlebitis. In addition, vancomycin has a potential for nephrotoxicity and ototoxicity, especially in patients with poor renal function and/or immune deficiency to bacterial infections. Hence, therapeutic drug monitoring (measurement of medication concentrations in blood) is warranted in patients with concomitant aminoglycoside therapy, patients on hemodialysis, patients with (potentially) altered pharmacokinetic parameters, patients administered high-dose or prolonged treatment, and patients with impaired renal function. Vancomycin has traditionally been considered a nephrotoxic drug. However, subsequent reviews on vancomycin-related nephrotoxicity suggest that many of the patients had also received other known nephrotoxins, especially aminoglycosides, and most of the rest had other confounding factors. In essence, the reputation of vancomycin as a nephrotoxin may be overstated.
Teicoplanin is a semisynthetic glycopeptide antibiotic with a spectrum of activity similar to vancomycin. Recently, it has been used for prophylaxis and treatment of serious infections caused by Gram-positive bacteria including methicillin-resistant S. aureus and Enterococcus faecalis. It is administered via IM route and has a much longer half-life (70–100 h) than vancomycin.
The quinolones are a family of synthetic broad-spectrum antibiotics. They prevent bacterial DNA from unwinding and duplicating. The majority of quinolones in clinical use belong to the subset fluoroquinolones, which have a fluorine atom attached to the central ring system (Ciprofloxacin, Levofloxacin, and Trovafloxacin). Fluoroquinolones are broad-spectrum antibiotics (effective for both gram-negative and gram-positive bacteria) used against serious bacterial infections, especially hospital-acquired infections and those resistant to other antibiotic classes. First- and second-generation fluoroquinolones selectively inhibit the topoisomerase II ligase domain, whereas third- and fourth-generation fluoroquinolones are more selective for topoisomerase IV ligase domain. Thus, they have enhanced gram-positive coverage. In general, fluoroquinolones are well tolerated, with most side effects being mild to moderate. Common side effects include gastrointestinal effects such as nausea, vomiting, and diarrhea, as well as headache and insomnia. Quinolones are contraindicated for patients with Q-T prolongation, pre-existing CNS (central nervous system) lesions or inflammation, epilepsy, or if the patient has suffered a stroke. Quinolones are also associated with an increased risk of tendinitis and tendon rupture in all age groups. The most severe form of tendinous disorder associated with fluoroquinolones is tendon rupture, which is common in the Achilles tendon. Resistance to quinolones can emerge rapidly, even during a course of treatment. Today, pathogens such as S. aureus, S. enterococci, and S. pyogenes exhibit resistance worldwide.
Aminoglycoside antibiotics are traditional Gram-negative antibacterial therapeutic drugs and contain a portion of the molecule as an amino-modified glycoside. Aminoglycosides exhibit bactericidal activity against gram-negative aerobes and some anaerobic bacilli. Kanamycin A, Tobramycin, Amikacin, Gentamicin, Streptomycin, Netilmicin, and Streptomycin are from this class of antibiotics. Aminoglycosides show concentration-dependent bactericidal activity. Aminoglycoside presence in the cytosol generally disrupts peptide elongation at the 30S ribosomal subunit, resulting in a rise of inaccurate mRNA translation and biosynthesis of proteins. Afterward, aminoglycosides can affect cell membranes and functional integrity of the bacterial cell membrane can be lost.
Clindamycin is recommended for prophylaxis in patients with penicillin allergy. It is from the lincosamide class and has primarily bacteriostatic effect. It inhibits ribosomal translocation and disrupts protein synthesis of the bacteria. It is most effective against infections involving aerobic Gram-positive cocci, including some members of Staphylococcus and Streptococcus (e.g., pneumococcus) and anaerobic, Gram-negative rod-shaped bacteria, including some Bacteroides, Fusobacterium, and Prevotella (resistance is increasing in Bacteroides fragilis). It is primarily used to treat infections caused by susceptible anaerobic bacteria. In addition, it is used to treat bone and joint infections especially those caused by S. aureus. Common adverse drug reactions (found in over 1 % of people) associated with systemic clindamycin therapy include diarrhea, vomiting, pseudomembranous colitis, abdominal pain, or cramps and/or rash and nausea.
Rifampicin or rifampin is a bactericidal antibiotic from the rifamycin class. It inhibits bacterial DNA-dependent RNA synthesis by inhibiting bacterial DNA-dependent RNA polymerase. Rifampicin is a more stable, highly efficient, and easy-to-tolerate semisynthetic derivative of the first original molecule, rifamycin. Rifampicin is typically used to treat Mycobacterium infections, including tuberculosis and leprosy. Rifampicin in combination with fusidic acid can be used in the treatment of methicillin-resistant S. aureus (MRSA) including infections such as osteomyelitis and prosthetic joint infections that are difficult to treat.
Fusidic acid is derived from the fungus Fusidium coccineum. Fusidic acid is a bacteriostatic antibiotic and is often used topically but may also be administered systemically as tablets or injections. Recently, the global emergence of antimicrobial resistance has led to a renewed interest in its use. Fusidic is bacteriostatic, in other words it inhibits bacterial replication and does not kill the bacteria. It inhibits bacterial protein synthesis by preventing the turnover of elongation factor G (EF-G) from the ribosome. It is active mainly against Gram-positive bacteria such as Staphylococcus species, Corynebacterium species, and Streptococcus species.
Prophylaxis
Infection is a major complication in orthopedic surgery since treatment is both difficult and expensive, and it may lead to serious sequelae. Prophylaxis of any bone infection is much more effective and easier than trying to treat it. Many studies suggest that antibiotic prophylaxis has been effective to reduce infection rates in orthopedic surgery. Infection in orthopedic surgery is strongly associated with the number of colonized bacteria in the first 24 h. The patient’s immune system reduces the number of colonized bacteria principally in the first 2 h. As the quantity of bacteria continues to increase in the next 4 h, the immune system will be inadequate to gain control. Therefore, the first 6 h is referred to as the “golden period” in prophylaxis. Antibiotics reduce the number of bacteria geometrically while preventing their re-colonization. In this context, prophylactic utilization of antibiotics prolongs the golden period [5, 6]. The antibiotic used for prophylaxis in orthopedic surgery should be effective against common bacteria, safe to use, and bactericidal. Prophylaxis must be effective against S. epidermidis and S. aureus that are mostly present in skin flora. Today, treatment of S. epidermidis infections has become more challenging due to their increased resistance to antibiotics. First-generation cephalosporins have been widely used since they are effective against these bacteria, and are cheaper and non-toxic. Vancomycin is not recommended for the first line of prophylaxis; however, it can be used as an alternative in patients with allergic conditions (Fig. 38.4) [1, 7, 8].
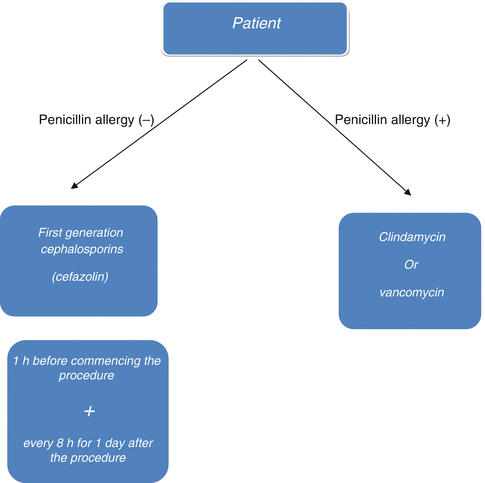

Fig. 38.4
Algorithm for the management of antibiotic prophylaxis in orthopedic surgery
Optimal timing for administration of antibiotics for prophylaxis is 30 min before incision. However, prophylactic antibiotics may be administered within the limit of up to 2 h prior to incision. Maximum dose should be administered and must be re-administered every 4 h of surgery-time or for each 1,000–1,500 cc of hemorrhage. Optimal period for prophylactic utilization of antibiotics is 24 h postoperatively and prolongation is not recommended due to the possible serious side effects of antibiotics [5, 8, 9].
Utilization of antibiotics in open fractures diverges from other “clean” procedures in orthopedic surgery. Since the skin barrier will be impaired after open fracture, bacteria will be in direct contact with bone tissue and be able to colonize much more easily. In 2004, a Cochrane systematic review reported that administration of antibiotics (first-generation cephalosporin) concurrent with proper wound debridement and effective aftercare has been highly effective in open fractures [10]. Antibiotic usage protocol in adults with open fractures is based on the Gustilo classification. First-generation cephalosporins are administered in type I and II open fractures with an initial dosage of 2 g and additional 1 g every 6–8 h for the following 48–72 h. In type III open fractures aminoglycosides with a dose of 3–5 mg/kg/day are administered for 48–72 h in addition to first-generation cephalosporins. If an organic contamination is suspected, an additional administration of 10–12 million units/day of crystallized penicillin is highly recommended [11]. In patients with fecal contamination who also have an allergic condition to penicillin, 500 mg of metronidazole administered every 6 h is recommended. Antibiotics used in open fractures of children are the same, but their dosages vary. In type I and II open fractures, first-generation cephalosporins of 100 mg/kg/day every 8 h are used without exceeding a daily total of 6 g. Aminoglycosides are used in the treatment of type III open fractures with a dosage of 7.5 mg/kg/day, in addition to the treatment protocol explained for type I and II open fractures. If organic contamination is suspected in children, crystallized penicillin (150,000 unit/kg/day) is used every 6 h, without exceeding a total dose of 24 million units. During the use of aminoglycosides, the patient should be checked regularly for ototoxicity [12].
Osteomyelitis
Osteomyelitis can be defined as any form of inflammation involving bone and/or bone marrow. Osteomyelitis is one of the most challenging conditions encountered by orthopedic surgeons. In the early twentieth century, 20 % of patients with osteomyelitis lost their lives, while others had major sequelae. Today, mortality and morbidity rates due to osteomyelitis are much lower due to the help of new antibiotics and aggressive surgical therapy.
Classification of osteomyelitis is according to the mechanism of infection or the patient’s response to treatment. Osteomyelitis is classified as acute, subacute, or chronic. However, there are no distinct borders in this classification, which describes the duration of the symptoms. The mechanism of the infection can be either exogenous or hematogenous. Orthopedic surgeons mostly deal with infections of exogenous osteomyelitis, which can be caused by open fractures, surgical intervention (after utilization of implants), or by local infections that can subsequently involve bone. Most cases of hematogenous osteomyelitis are caused by bacterial pathogens. In addition, osteomyelitis is classified as pyogenic or non-pyogenic depending on the patient’s response.
Acute osteomyelitis is defined as an infection diagnosed within 2 weeks of the onset of symptoms. Acute hematogenous osteomyelitis typically involves the metaphysis of long tubular bones, with approximately two-thirds of all cases involving the femur, tibia, or humerus, especially in children. The principal cause of osteomyelitis in children is S. aureus (70–90 %) and unfortunately, globally emerging meticillin-resistance among S. aureus has changed both the epidemiology and treatment options. Other etiological microorganisms include S. pneumonia, S. pyogenes, coagulase-negative Staphylococci (especially in implant-associated infections), Group B Streptococci (in infants), Kingella kingae, enteric Gram-negative bacilli (especially Salmonella spp. in patients with sickle cell disease), and anaerobic bacteria. If the etiologic agent of osteomyelitis is identified, specific antimicrobial treatment based on the susceptibility profile of the organism should be given. The treatment of acute hematogenous osteomyelitis requires appropriate antimicrobial therapy in all cases and may require surgical incision and drainage. Whenever an abscess (intra-osseous, subperiosteal, and/or soft-tissue) exists, incision and drainage should be performed. If MRSA is not a concern, empiric therapy for children (older than 3 months) should be an anti-Staphylococcal penicillin (nafcillin and oxacillin) or first-generation cephalosporin (cefazolin). If MRSA is suspected, vancomycin or clindamycin can be used for treatment.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








