Chapter 12 Shoulder and Elbow Arthroplasty
Reconstructive Procedures of the Shoulder
History
The earliest known report of shoulder arthroplasty dates back to 1893, when a French surgeon, Péan, substituted a platinum and rubber implant for a glenohumeral joint destroyed by tuberculosis (Fig. 12-1). In the early 1950s, Neer introduced a humeral head prosthesis that he planned to use for complex shoulder fractures. In 1951, he reported his initial results of replacement of the humeral head with an unconstrained cobalt-chromium alloy (Vitallium) prosthesis. In 1974, the Neer II humeral prosthesis, which was modified to conform to a glenoid component, was introduced. Total shoulder arthroplasty using a constrained articulated unit in patients with loss of the rotator cuff but with a functional deltoid muscle was popular in the early 1970s but had limited success and has been abandoned.
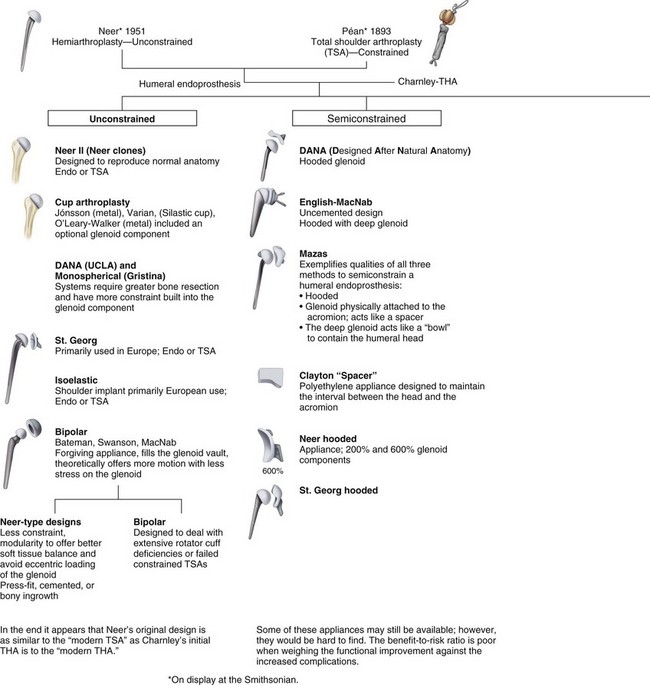
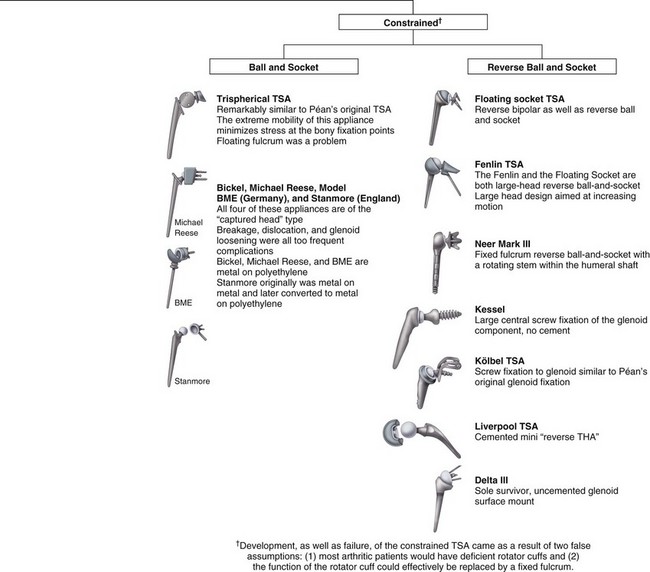
FIGURE 12-1 Family tree of shoulder arthroplasty prostheses.
(Adapted from Gross RM: The history of total shoulder arthroplasty. In Crosby LA, editor: Total shoulder arthroplasty, Rosemont, IL, 2000, American Academy of Orthopaedic Surgeons.)
Anatomy and Biomechanics
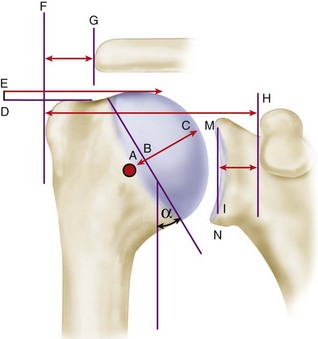
Restoration of glenohumeral anatomy is essential for a good functional outcome. Anatomical studies have defined the humeral geometry further and suggested applications to shoulder arthroplasty prosthesis design and surgical techniques (Fig. 12-2 and Table 12-1). The articular surface of the humeral head is essentially spherical, with an arc of approximately 160 degrees covered by articular cartilage. The radius of curvature is approximately 25 mm and is slightly larger in men than in women. The glenoid articular surface radius of curvature is 2 to 3 mm larger than that of the humeral head. The average neck-shaft angle is 45 degrees (±5 degrees), with a range of 30 to 50 degrees. Murthi et al. found that arthritic shoulders have a flatter neck-shaft angle close to 50 degrees. CT studies found that the normal position of the glenoid surface in relation to the axis of the scapular body ranged from 2 degrees of anteversion to 7 degrees of retroversion.
TABLE 12-1 Anatomical Characteristics of the Shoulder Important for Prosthesis Design
| Glenoid diameter | |
| Superior anteroposterior | 18-30 mm |
| Inferior anteroposterior | 21-35 mm |
| Superoinferior (height) | 30-48 mm |
| Inclination | |
| Glenoid | Average 4.2 degrees (−7 to 20 degrees) |
| Humeral head | 30-55 degrees |
| Version | |
| Glenoid | 1.5 degrees retroversion (10.5 to 9.5 degrees anteversion) |
| Humeral head | 0-55 degrees retroversion (dependent on measurement method; highly variable among individuals) |
| Surface area | |
| Glenoid | 4-6 mm |
| Humeral head | 11-19 mm |
| Cartilage thickness | |
| Glenoid | 2.16 mm |
| Humeral head | 1.44 mm |
| Radius of curvature | |
| Glenoid | 22-28 mm |
| Humeral head | 23-28 mm (smaller in women than men) |
| Humeral offset | |
| Medial (coronal) | 4-14 mm |
| Posterior (transverse) | −2 to 10 mm |
| Head-shaft angle | 30-55 degrees |
The superior margin of the humeral head articular surface normally is superior to the top of the greater tuberosity by 8 to 10 mm (see Fig. 12-2). Restoring the center of rotation for the humeral head in relation to the axis of the humeral diaphysis may play a role in prolonging glenoid fixation and decreasing polyethylene wear. The distance from the lateral base of the coracoid process to the lateral margin of the greater tuberosity is called the lateral humeral offset. Maintaining this distance is important because a significant decrease reduces the lever arms for the deltoid and supraspinatus muscles, which weakens abduction and impairs function. A significant increase causes excessive tension on the soft tissues (“overstuffing” of the joint), which also results in loss of motion. A biomechanical cadaver study determined that humeral articular malposition of more than 4 mm led to increased subacromial contact and that offset of 8 mm in any direction significantly decreased passive range of motion. The authors suggested that anatomical reconstruction of the humeral head/humeral shaft offset should be within 4 mm of normal to minimize subacromial contact and maximize glenohumeral motion.
Prosthesis Design
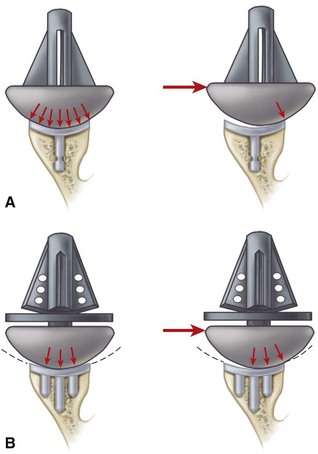
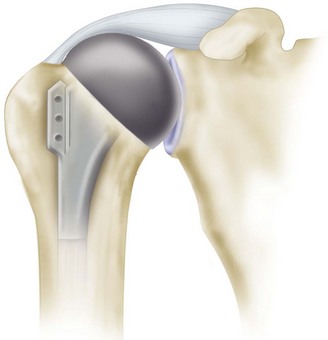
Cemented all-polyethylene components remain the most frequently used glenoid components, but most now have an increased radius of curvature compared with the humeral head (2 to 6 mm larger) to allow translation during movement and to decrease edge loading. Several studies have shown that translation accompanies glenohumeral rotation after total shoulder arthroplasty. Such translation in a perfectly congruent joint may have a potential for localized wear and loosening (rocking-horse effect); however, increased loosening and polyethylene have not been reported to occur when the radii of curvature of the glenoid component and the humeral head are matched within 2 mm. In a multicenter study of 319 total shoulder arthroplasties using the same type of prosthesis, Walch et al. noted fewer radiolucencies with mismatches between the glenoid and humeral head diameters of more than 5.5 mm (6 to 10 mm). They cautioned that the upper limit of mismatch has not been conclusively determined, and thus greater prosthetic mismatches could lead to increased joint translation, accelerated polyethylene wear, and fracture. Current opinion seems to suggest that a glenoid with a radius curvature of 2 to 4 mm larger than the humeral head allows normal translation during rotation without rim loading or risk of loosening (Fig. 12-3).
Clinical Presentation and Radiographic Evaluation
The radiographic appearance varies with the patient’s pathological process. Those with osteoarthritis reliably demonstrate subchondral sclerosis and a large osteophyte on the inferior aspect of the humeral head (Fig. 12-4). This so-called “goat’s beard” is pathognomonic of advanced glenohumeral degeneration. These osteophytes can enlarge the humeral head to twice its normal size, resulting in capsular distention. Posterior instability caused by this capsular distention and posterior glenoid erosion may require capsular reefing or bone grafting at the time of shoulder arthroplasty. This is most common in cases of capsulorrhaphy arthropathy. Joint space narrowing, which is so reliably seen in hip and knee osteoarthritis, is not commonly seen in the shoulder until very late in the disease process owing to the non–weight-bearing position of the shoulder under standard radiography. Axillary lateral radiographs typically demonstrate posterior subluxation of the humeral head on the glenoid, and a wear pattern in the posterior glenoid may be present (Fig. 12-5). This same pattern is most often seen in advanced cases of posttraumatic arthritis and osteonecrosis. Patients with capsulorrhaphy arthropathy have a similar radiographic appearance except that loose bodies and osteophytes tend to be more common and numerous (Fig. 12-6) than in standard osteoarthritis.
FIGURE 12-6 Radiograph showing capsulorrhaphy arthropathy; note numerous loose bodies and osteophytes.
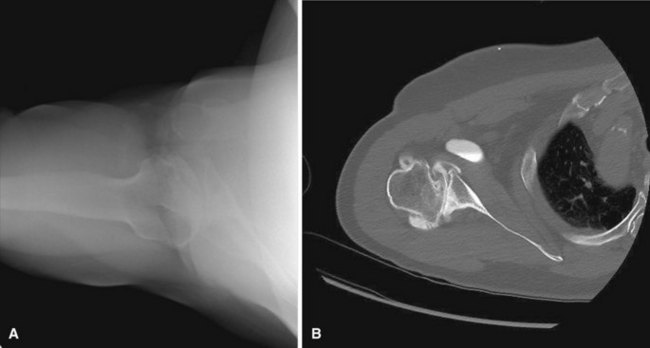
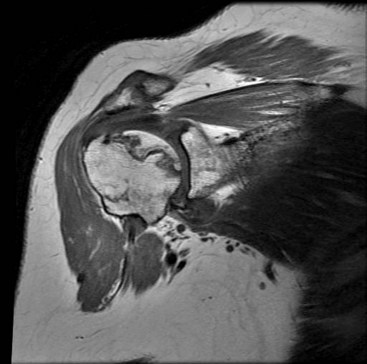
MRI may be a useful adjunct in this population. In patients with strength deficits that could be due to either arthritic pain or a torn rotator cuff, MRI can help determine the status of the tendons. Whereas rotator cuff tendinopathy is common in this setting, full-thickness tears are uncommon and are seen in only about 10% of patients. MRI also typically demonstrates advanced cartilage degeneration and may show numerous other findings, including thinning of the subscapularis and degenerative changes in the biceps tendon. Increased capsular volume posteriorly and capsular contraction anteriorly are usual changes as well. Finally, in patients with pre-collapse osteonecrosis, MRI is useful to visualize the area of dead bone and is often the best tool to make the diagnosis (Fig. 12-7).
Hemiarthroplasty
Surgical Technique
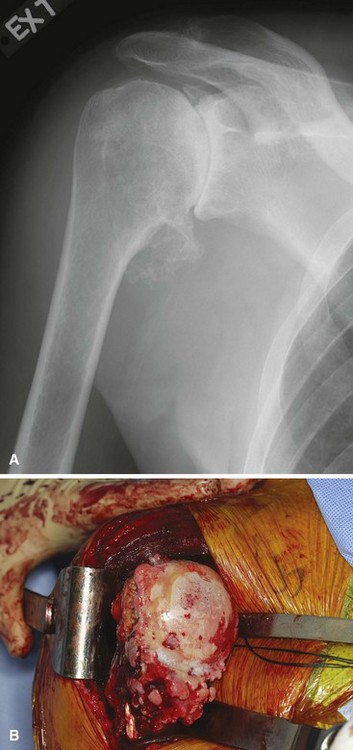
The goal of hemiarthroplasty is restoration of the humeral articular surface to its normal location and configuration. Because the glenoid is not replaced, the size, radius, and orientation of the prosthetic joint surface must duplicate that of the original biological humeral head. Radiographs of the contralateral shoulder can provide information about a patient’s normal humeral head anatomy. Care should be taken to avoid a “big head” humeral prosthesis that can “overstuff” the joint (Fig. 12-8).
Hemiarthroplasty
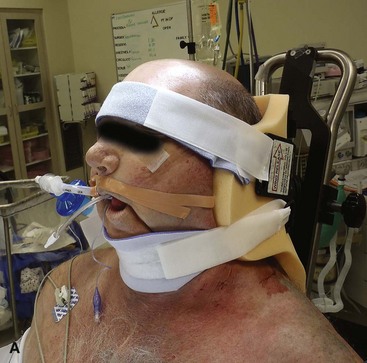
• Place the patient in the beach chair position using a McConnell headrest (McConnell Orthopaedic Equipment Company, Greenville, TX) to allow positioning of the patient at the top and edge of the table (Fig. 12-9A). Pad all bony prominences. The medial border of the scapula should be free and off the table, allowing full adduction to gain access to the intramedullary canal.
• Secure the patient’s head to the headrest, holding the head in a position that avoids hyperextension or tilting of the neck, which can cause compression of the cervical roots.
• Prepare the arm and drape it widely. We recommend using occlusive dressings to cover the entire surgical field because of the risk of contamination from the axilla.
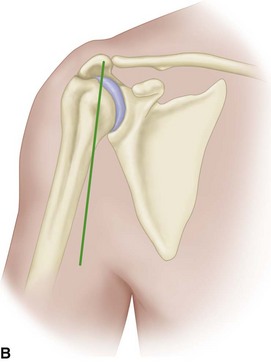
• Make an incision anteriorly, approximately halfway between the coracoid and the lateral aspect of the acromion (Fig. 12-9B). Carry dissection down to the deltoid and raise medial and lateral flaps to mobilize the deltoid.
• Open the deltopectoral interval and allow the cephalic vein to fall medially.
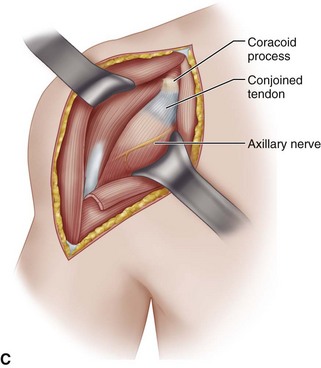
• Perform subdeltoid, subcoracoid, and subacromial releases to release the proximal humerus. In the subcoracoid space, locate the axillary nerve by passing the volar surface of the index finger down along the anterior surface of the subscapularis muscle (Fig. 12-9C). If scarring and adhesions make identification of the nerve difficult, pass an elevator along the anterior surface of the subscapular muscle to create an interval between the muscle and the nerve. Always identify the axillary nerve and carefully retract and hold it out of the way, especially during the crucial steps of releasing and resecting the anteroinferior capsule.
• Incise the subscapularis 1 cm medial to the lesser tuberosity. Place two retention sutures in the subscapularis to be used as traction sutures when freeing the rest of the tendon from the underlying capsule and scar tissue. At closure, use the sutures to repair the tendon.
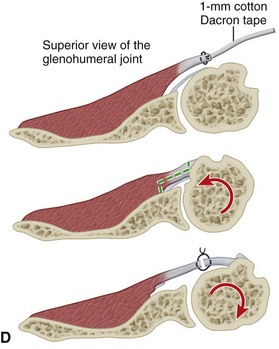
• Some authors prefer either a lesser tuberosity osteotomy or a release of the subscapularis directly off of bone. If external rotation is markedly limited, the subscapularis also can be reattached to the proximal humerus more medially to allow increased external rotation. Alternatively, the tendon can be lengthened with a coronal Z-plasty technique (Fig. 12-9D and Table 12-2).
• Incise the rotator interval, directing the cut medially toward the glenoid. Typically, a large amount of synovial fluid escapes as the joint is entered.
• Release the anteroinferior capsule from the humerus, and externally rotate the arm to bring the inferior aspect of the shoulder capsule into view. If osteophytes are present inferiorly on the humeral head, remove them to expose the capsule more fully. Take care to stay directly on bone so as not to injure the axillary nerve during the capsular release. The importance of the inferior capsule release cannot be overstated and must be thoroughly carried out to at least the 6 o’clock position to dislocate the humeral head and gain access to the glenoid.
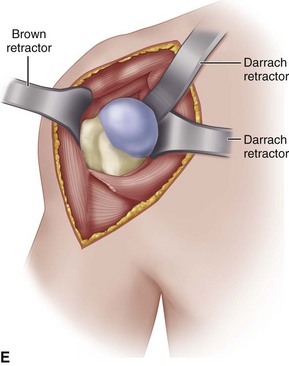
• Once the capsule is adequately released, place a large Darrach retractor in the joint and gently externally rotate, adduct, and extend the arm to deliver the humeral head up and out of the glenoid fossa (Fig. 12-9E). If the humeral head cannot be delivered in this fashion, the inferior capsule must be released further.
• Prepare the humeral canal, using the humeral axis to reference the osteotomy. Initially, open the canal with a high speed burr at the base of the rotator cuff footprint and ream it to a size where appropriate “chatter” is felt in the shaft. Do not use motorized equipment for reaming, and be careful not to overream the canal, which could create a stress riser or cause a fracture.
• We prefer to use a cutting guide that employs extramedullary referencing, using the axis of the forearm as the reference point. With the cutting guide pinned into position at 30 degrees of retroversion, recheck the cutting angle and confirm that the height is such that the saw will not violate the rotator cuff or biceps tendon.
• Complete the osteotomy with an oscillating saw. If any inferior humeral head osteophyte remains, remove it with a rongeur.
• After the head cut, broach the humeral canal to the same size as the reamed canal. It is imperative to confirm proper position of the broaches in 30 degrees of retroversion during this step to prevent component malposition.
• Inspect the glenoid to confirm there is enough glenoid cartilage to provide an adequate bearing surface for the metal humeral head. After this inspection, check the humeral trial stem to ensure it is seated securely within the humeral canal. If so, tap the component stem into position, taking care to keep the stem in 30 degrees of retroversion.
• If cementing is deemed necessary because of a previous surgical procedure, fracture, osteoporosis, rheumatoid arthritis, or degenerative cysts, place a cement restrictor or a cortical bone plug from the resected humeral head 2 cm inferior to the tip of the prosthesis.
• Place a trial humeral head and reduce the glenohumeral joint using internal rotation and gentle traction. With the arm in neutral rotation, check the height of the humeral head to confirm anatomic reconstruction. As a rule of thumb, the most superior aspect of the humeral head should be 1 cm superior to the greater tuberosity.
• Also check the version to confirm that the humeral head rests directly across from the glenoid. With a thumb on the lesser tuberosity, push the humeral head posteriorly and then release it: 30% to 50% posterior excursion with immediate “snap back” of the humeral head is optimal. Evaluate forward elevation and internal rotation.
• Some authors recommend concomitant biceps tenodesis. If this is desired, it should be done before impaction of the humeral head.
• Once the checks have been performed, thoroughly clean the Morse taper, impact the humeral head into position, and reduce the joint for the final time.
• Perform a tight closure of the rotator interval as well as the subscapularis with heavy No. 2 suture. If the tendon was divided or lengthened, repair and secure it with heavy nonabsorbable sutures to allow immediate passive movement beginning the day after surgery. Place a drain in the deltopectoral interval and close it with No. 0 sutures. Close the skin in standard fashion and place the arm in a soft sterile dressing and a sling while the patient is still upright and before being aroused from anesthesia.
TABLE 12-2 Guidelines for Release of Subscapularis Tendon
| SUBSCAPULARIS TENDON RELEASE | PREOPERATIVE RANGE OF MOTION |
|---|---|
| Release 1.5 cm medial to insertion | Passive external rotation ≥20° |
| Release subperiosteally, reattach medially | Passive external rotation >−20° and <20° |
| Subscapularis Z-lengthening | Passive external rotation ≤−20° |
Data from Schenk T, Iannotti JP: Prosthetic arthroplasty for glenohumeral arthritis with an intact or repairable rotator cuff: indications, techniques, and results. In Iannotti JP, Williams GR Jr, editors: Disorders of the shoulder: diagnosis and management, Philadelphia, 1999, Lippincott Williams & Wilkins.
Outcomes
When used for the treatment of osteonecrosis, hemiarthroplasty has been reported to provide consistently good pain relief in 90% to 100% of patients, with an almost normal range of motion (Table 12-3). Results are not quite as good in patients with rheumatoid arthritis, osteoarthritis, or posttraumatic glenohumeral arthrosis but are satisfactory in most patients, although range of motion is decreased.
Modified Hemiarthroplasty—interposition Arthroplasty and Glenoidplasty (Ream and Run)
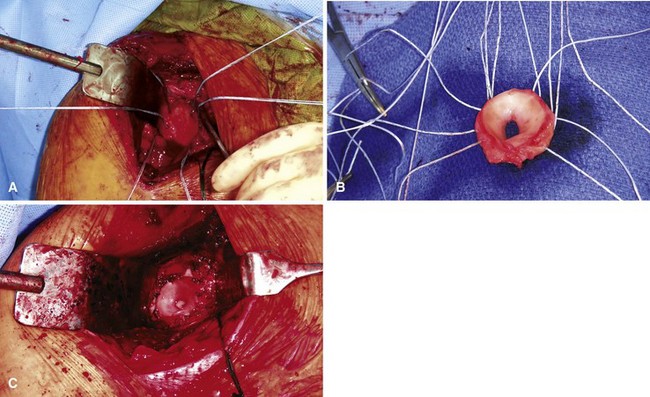
Recently, lateral meniscal allografts have been used as an interposition material. In this procedure, the anterior and posterior horns of the allograft are sewn to each other to form a circular surface for articulation with the humeral head. The allograft is then laid onto the glenoid and secured, typically with suture anchors (Fig. 12-10). Significant improvements in pain and function have been reported with this procedure. Although joint space narrowing did occur, glenoid erosion did not progress, suggesting that the lateral meniscus may offer some protection against glenoid wear.
Resurfacing Hemiarthroplasty
With the success of humeral hemiarthroplasty, other techniques have been developed to allow less invasive replacement of the proximal humerus but also to preserve bone stock. These humeral resurfacing procedures do not use a stem for intramedullary fixation but instead form a cap over the humeral articular surface and are typically stabilized with a smaller post in the metaphysis (Fig. 12-11). Outcomes of humeral resurfacing have generally been successful, with patient satisfaction rates as high as 93% and overall results similar to that of stemmed prostheses. Several series have reported similar success in specific patient cohorts with rheumatoid arthritis, osteoarthritis, and cuff tear arthropathy. An average operative time of 40 minutes was reported for humeral resurfacing in a cohort of patients older than 80 years of age, with no perioperative deaths or other serious medical complications, and in a young group of patients (average age 42 years) there were significant improvements in pain and functional scores and all but one patient returned to full activity.
Total Shoulder Arthroplasty
Surgical Technique
Total shoulder arthroplasty
• Approach the glenohumeral joint as described in Technique 12-1. Once the trial broach is tapped into position, remove the retractors from about the humerus.
• Expose the glenoid by placing a Fukuda retractor on the posterior aspect of the glenoid and subluxing the humerus posteriorly.
• Débride the glenoid vault of all remaining labral tissue and articular cartilage.
• If needed for exposure, release the anterior capsule and place a flat Darrach retractor on the anterior glenoid neck to aid exposure.
• The glenoid is not adequately exposed until the anterior, posterior, superior, and inferior aspects of the glenoid can be seen. Once this is accomplished, inspect the glenoid for wear and bone defects. Typically, there is posterior erosion of the glenoid and the anterior rim of the glenoid needs to be lowered to reestablish correct version (Fig. 12-12). This can be done by eccentric reaming or with a high-speed burr. A preoperative CT scan can aid in understanding glenoid orientation and morphology.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree