Fig. 5.1
A thumb replantation. An amputated thumb (a) at injury, (b) 2 months and (c) 8 months following replantation, showing good functional recovery (Courtesy of G. Chick, MD)
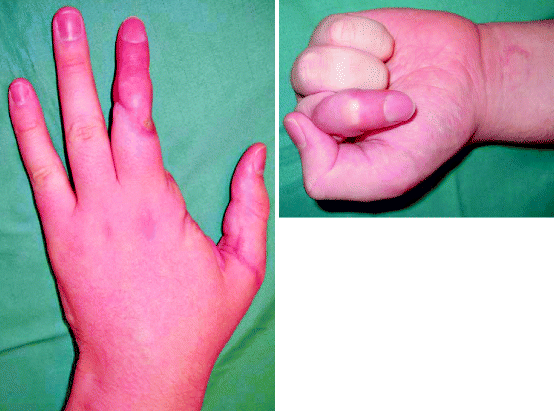
Fig. 5.2
A revascularised thumb, a ray amputation of the index finger and replantation of the middle finger, 6 months post-injury. The thumb has recovered sensation and range of movement extremely well. The ray amputation of the index finger has opened up the first web space and does not attract the eye. The replanted middle finger has a good range of movement at the distal interphalangeal joint and metacarpophalangeal joint but has a fused proximal interphalangeal joint. Although the circulation is adequate and the finger has protective sensation, the finger looks different to the ring and little and has a withered pulp. Despite this, the patient has returned to work and is using their hand
Any parts amputated by the injury should be collected if possible and sent to the hospital with the patient. Even if these bits cannot be formally replanted, they may provide useful ‘spare parts’ which improve the final functional outcome.
The patient and amputated part need to be transferred to the microsurgical centre as soon as possible. Proximal hand or arm amputations contain muscle which has a cold ischaemia time of less than 12 h. Fingers do not contain muscle and tolerate ischaemia for longer periods of time. There are reports of fingers being successfully replanted after more than 24 h of cold ischaemia. Despite this, the replantation is best done as soon as possible to minimise the ischaemic time and therefore the tissue damage.
Careful transfer of the amputated parts increases the chance of success if a replantation is undertaken. In order to preserve the tissues for as long as possible, the amputated part needs to be chilled during transfer to the microvascular centre. The amputated parts should be wrapped in a moist gauze dressing and placed into a sealed plastic bag to stop desiccation. They are then chilled by putting this plastic bag into a bag or container of ice and water (Fig. 5.3). The slurry of ice and water keeps the tissue at around 4° Celsius, which preserves it perfectly. If the part is placed onto dry ice, or into a bag of frozen peas, the tissue freezes to the ice. This frostbite damage to the amputated part usually means that it cannot be replanted successfully.


Fig. 5.3
How to transfer an amputated body part
It is sensible to clearly label the container of body parts with the patients name as it is not unheard of for more than one patient to arrive at the same time with amputations.
If any part of the hand is dangling on a piece of attached tissue, no matter how small, it should be left attached. Although tissues tolerate warm ischaemia for less time, do not be tempted to complete the amputation in order to formally chill the distal part. Any piece of tissue connecting the part to the body may have a small blood vessel which could make the difference between survival or loss following a replantation. The loose body parts should be gently included in the dressing, trying to lay them straight so that any residual circulation can flow.
Key Points
Life before limb.
Elevate the hand and remove all rings.
Direct compression to control bleeding.
Apply a supportive dressing and consider whether a splint would be helpful for analgesia.
Wrap amputated parts in moist swabs, put them in a water tight container and then place that in a slurry of ice and water. Put the patient’s name on this and send with the patient urgently to a microsurgical hand surgery department.
5.2 Acute Compartment Syndrome
Acute compartment syndrome occurs when the circulation to muscles is reduced or stopped due to an increase in pressure within their fixed volume fascial compartment. The forearm and hand have a number of fascial compartments containing muscles as do the lower limbs. Although containing muscles within these fixed fascial compartments increases their mechanical efficiency, it makes their circulation vulnerable after certain types of injury. Crushing injuries are a common cause of compartment syndrome, as is the return of blood flow following repair of an arterial injury. Other injuries which can result in an acute compartment syndrome include fractures, lying on a limb for a long period, high-pressure injection injuries and burns. Young men are most at risk of developing a compartment syndrome after an injury, possibly due to their relatively high muscle mass [3].
The swelling which occurs following these injuries is constrained by the sealed fascial compartments containing muscles and so the tissue pressure, which is usually very low, rises. When the tissue pressure inside the compartment rises to within 10–30 mmHg of the diastolic blood pressure, the local blood flow becomes inadequate causing muscle damage. When the tissue pressure of the compartment reaches diastolic blood pressure, local blood flow ceases [5]. The muscles will die if the pressure is not released in around 2 h following the onset of the ischaemia. Acute compartment syndrome is a surgical emergency.
The consequences of a missed compartment syndrome are so devastating that it is better to err on the side of caution and perform fasciotomies if an acute compartment syndrome is suspected.
5.2.1 Diagnosis
Compartment syndrome is usually a straightforward clinical diagnosis. The most important point is to have a clinical suspicion that a particular injury can result in compartment syndrome and to therefore monitor the patient closely. Traditionally, acute compartment syndrome is diagnosed by the four P’s of pain, paraesthesia, paralysis and pulselessness.
The pain of compartment syndrome characteristically is out of proportion to the injury. The pain is relentless and progressive. It is not helped by immobilisation and is significantly worse if the muscles within the affected compartment are stretched. This is the most sensitive and usually the first diagnostic finding. The affected muscle compartments are tense on palpation. Altered sensation and muscle weakness may be found once nerve and muscle ischaemic damage has begun. The loss of a peripheral pulse is a late finding, and the diagnosis should be made before this point.
The tissue pressure within a compartment can be measured by placing a pressure transducer into that compartment [4]. This is a helpful adjunct to a clinical diagnosis if it can be performed without delaying treatment. Certain conditions need to be met. Firstly, the tip of the transducer needs to be accurately placed in the correct compartment. This can be difficult in the small compartments of the hand. Secondly, the measurement should be related back to the patient’s blood pressure and not considered in isolation. A low diastolic blood pressure, often found in athletes, could mean a lower intra-compartment pressure can be consistent with compartment syndrome. A differential pressure of less than 30 mmHg between the diastolic blood pressure and compartment pressure means that an acute compartment syndrome has developed and a fasciotomy should be performed [2].
It is quite appropriate to make a diagnosis of an acute compartment syndrome on a clinical basis alone and proceed immediately to surgery.
5.2.2 Treatment
Once the diagnosis of a compartment syndrome has been reached, the muscle compartments need decompressing urgently to restore their circulation and prevent permanent ischaemic damage.
Fasciotomies are usually performed through long skin incisions as the skin envelope itself can be tight enough to have a compromised circulation following a crush injury. The fascia surrounding the different muscle compartments is then released. The incisions are planned to allow access to all the compartments whilst minimising damage to cutaneous nerves and allowing the major nerves to the hand to be covered by skin flaps and protected in the post-operative period [1].
Two dorsal incisions on the hand are used to decompress all four web space interossei compartments in the dorsal and palmar aspects of the hand. The hypothenar muscles are decompressed through the midlateral incision on the ulnar aspect of the hand and the thenar muscles through the similar incision on the radial side of the hand. The median nerve beneath the tight transverse carpal ligament is decompressed as part of the volar forearm incision. The ulnar nerve in Guyon’s canal is also released. The superficial and deep groups of the forearm flexor muscles are released using this incision. A straight incision along the dorsal aspect of the forearm will give access to the extensor muscles to the fingers and the mobile wad muscles (Fig. 5.4).
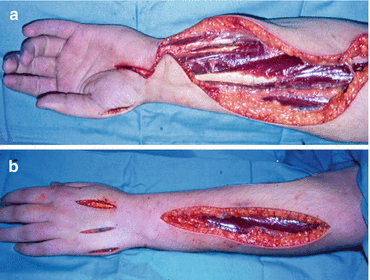

Fig. 5.4
A fasciotomy operation showing the incisions to decompress the volar forearm muscles, carpal tunnel and thenar eminence muscles (a) and the dorsal forearm muscles, intrinsic muscles and hypothenar eminence muscles (b)
Which muscles need decompressing is determined both clinically before starting the procedure and also by the findings during the operation (Fig. 5.5). Fasciotomies may also be performed prophylactically during the initial surgical treatment of severe hand injuries to prevent the development of compartment syndrome.
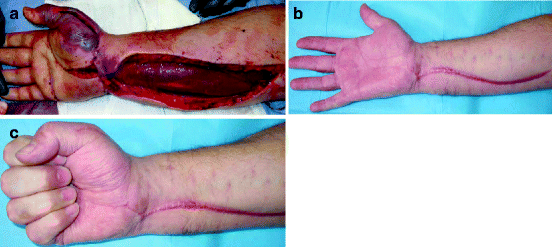

Fig. 5.5
Acute compartment syndrome. (a) Following release of the volar forearm, the flexor muscle mass is seen bulging out of its fascial compartment. At this moment, the muscle colour has improved from the blue seen through the unreleased fascia, but has not returned to a healthy red. (b, c) Three months later, an excellent range of movement has been regained. The carpal tunnel and thenar eminence release scars have healed inconspicuously but the volar forearm scar is hypertrophic as is often the case
The fingers do not usually need releasing as they do not contain muscles. Occasionally, the fingers are so swollen that their circulation is also compromised. In this case, an escharotomy type of incision can be made along the midlateral line of the nondominant border of the digit, for example, the ulnar border of the index finger and the radial border of the thumb and little finger. The nondominant borders of the central two digits depend on how the hand is used. It is usually the ulnar border of each finger, but this may need to be changed after discussion with the athlete.
Once the muscles have been decompressed, the skin wound over the median nerve is loosely closed to protect the nerve from desiccation. The other wounds can be simply dressed and dealt within 48–72 h when the swelling following the injury has improved. They can be gently ‘boot laced’ with elastic vascular slings. This prevents as much separation of the skin edges but leaves the muscle compartments decompressed. The wounds can also be split skin grafted, either immediately or at a second operation.
The final scars following a split skin graft which is then serially excised at a later date may be cosmetically more acceptable than the scars achieved when the fasciotomy wounds are sutured closed as part of the initial treatment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








