Somatosensory system
The functions of the somatosensory system are:
- to monitor the contact of objects and surfaces with the skin, particularly the hands and feet;
- to report the position of body segments in space and in relation to each other (body scheme);
- to initiate sensory activity for the interpretation of harmful stimuli.
From this system, one knows where the arms are in space, the pressure of a pencil held in the fingers, and how cold the wind is on the face.
The skin is not only a simple sense organ for touch, but responds to the particular pressure and temperature of surfaces. In gripping, the feedback from all the receptors of the skin in contact with the object guides the muscle force that is required. Pressure receptors in the skin of the soles of the feet monitor the distribution of body weight over the feet, and therefore assist balance reactions.
In reaching out to grasp an object, the proprioceptors in the upper limb monitor the changing angulation of the joints as the movement proceeds. We can judge the weight of an object held in the hand from activity in the proprioceptors of the elbow flexors and the mechanoreceptors in the skin of the palm of the hand.
The sensory information from the somatosensory system forms the body scheme and regulates posture during movement. We are unaware of a large amount of the activity of the somatosensory system and its role in movement is often underestimated.
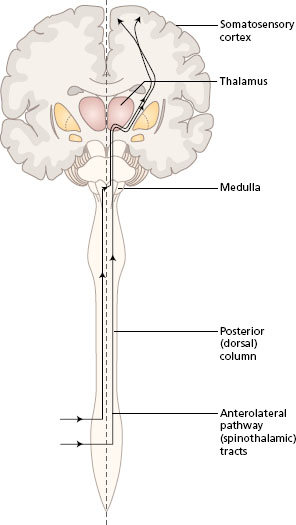
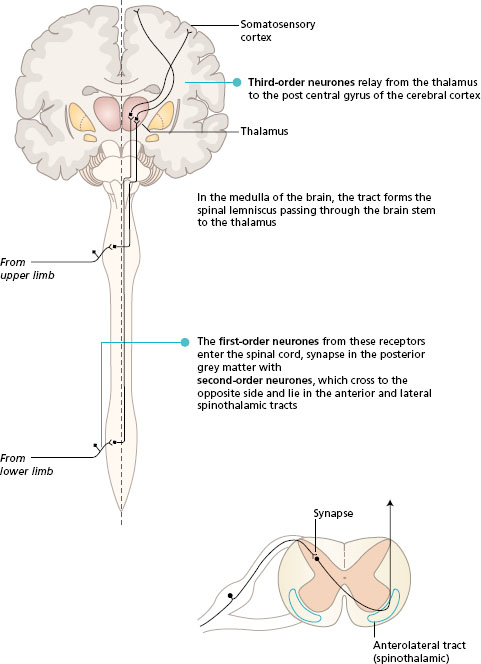
Somatosensory information is transmitted in two main ascending pathways in the spinal cord and the brain, known as the posterior (dorsal) column pathway and the anterolateral pathway. Alternative names are the medial lemniscus pathway and the spinothalamic tracts, respectively. Both systems link the receptors on one side of the body with the somatosensory area in the opposite parietal lobe. The anterolateral pathway crosses at the spinal level, while the posterior column crosses in the sensory decussation in the medulla of the brain. The pathways converge in the brain stem, and both synapse in the thalamus. All fibres from both the anterolateral and posterior column pathways pass through the internal capsule to end in the somatosensory area of the parietal lobe, where the body parts are represented in a particular topographical arrangement (see Chapter 3 ).
- Revise the composition of a spinal nerve from Chapter 4, and the position of ascending tracts of the spinal cord described in Chapter 3.
- Look at Figure 11.1 to follow the general plan of these two pathways.
The function of each of the two sensory pathways is different, even though some of the sensation transmitted appears to be the same. The posterior column pathway is concerned with fast -acting information that has a high degree of discrimination. For example, changes in joint position occur rapidly during movement. The changing activity in the joint proprioceptors is conducted via this route. The anterolateral pathway conducts the reponses from stimuli, such as temperature, that are neither urgent nor require precise location.
Figure 11.1 Ascending pathways to the somatosensory cortex, general plan. Frontal section of the brain with spinal cord.
Posterior (dorsal) column
The posterior column pathway provides the route for touch and proprioception. This ascending route also plays a part in the interpretation of pain. The important role of this ascending system is in the combination of input from more than one modality to interpret complex sensations. For example, both touch and proprioception are involved in the ability to distinguish the size and the shape of an object without vision.
The activity in the posterior column route is initiated by receptors that are fast adapting with large -diameter axons. These receptors are found in the skin and also lying in muscles, tendons and joints (proprioceptors). The sensory neurones enter the posterior horn of the spinal cord, and then pass into the posterior (dorsal) column of white matter of the same side. Many of the first-order neurones branch to synapse with interneurones in the posterior horn at the spinal level of entry. The posterior column of white matter, lying underneath the lamina of each vertebra, becomes larger as it ascends the spinal cord, collecting sensory fibres from each spinal nerve. In the medulla of the brain, the neurones end in the gracile and cuneate nuclei. At this level, the second -order neurones cross to the opposite side and pass through the brain stem in the medial lemniscus to the thalamus. The third-order neurones project to the somatosensory cortex.
The fibres in the posterior columns are ipsilateral, i.e. they carry sensation from the same side of the body. Fibres from the lower limbs are most medial and form the fasciculus gracilis. As more fibres enter the spinal cord from sacral to cervical segments they are added laterally. In this way, the fibres from the upper limb form the fasciculus cuneatus.
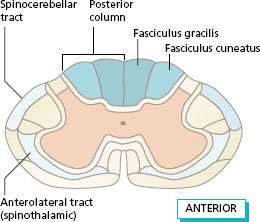
Figure 11.3 shows the position of the main ascending tracts in position in the spinal cord at the level of the cervical segments. A similar section at the level of the lumbar segments would have a smaller posterior column with no fasciculus cuneatus. The posterior spinocerebellar tract is formed by some of the fibres from proprioceptors that enter the lateral white matter and reach the ipsilateral cerebellum.
Anterolateral pathway (spinothalamic tract)
This pathway is primarily concerned with temperature and nociceptive sensations. The spinothalamic tracts play a supplementary role for touch sensation, but probably only become important when the posterior column is damaged.
Activity in the anterolateral pathway originates in sensory neurones with slowly adapting receptors in the skin. The sensory neurones have small -diameter axons with slow conduction velocity. These sensory neurones enter the spinal cord and synapse in the posterior horn before crossing to the opposite side to enter the spinothalamic tract. The fibres of the spinothalamic tract lie in the anterolateral white matter of the spinal cord. This route has been divided into anterior and lateral spinothalamic tracts, but more recent work has shown no difference in the spread of fibre types across the pathway. There is a topographical arrangement of the fibres in the anterolateral pathway, with those from distal body segments more lateral, and proximal areas more medial.
The anterolateral route continues in the brain stem, to end in the thalamus. The third -order neurones project from the thalamus to the somatosensory area in the parietal lobe.
Figure 11.2 Posterior (dorsal) column pathway seen in a frontal section of the brain with spinal cord, and its position in a transverse section of the spinal cord.
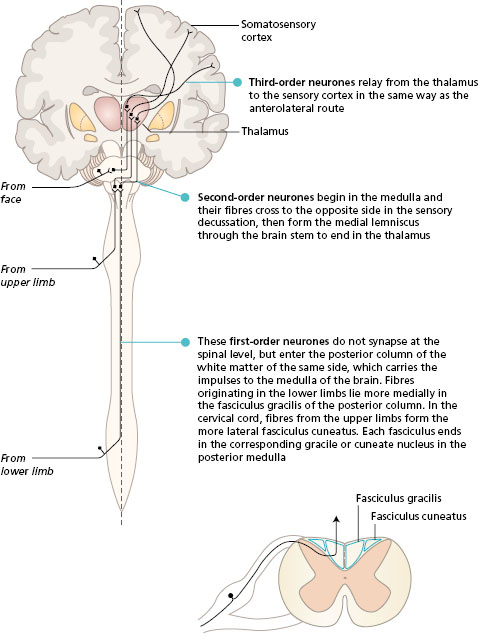
Figure 11.3 Position of the ascending tracts at the cervical level.
In the brain stem, some of the second -order neurones branch to link with the reticular formation.
Sensory information from the face
Receptors in the skin and the muscles of the face and in the mouth enter the brain stem mainly in the trigeminal (fifth cranial) nerve and synapse in the sensory nuclei of this nerve. Second -order neurones cross to the opposite side and lie alongside the medial lemniscus to reach the thalamus. Third -order fibres end in the region representing the face in the somatosensory cortex in the parietal lobe. Input from this trigeminal system is important for the sensory background to the movements of facial expression, swallowing and speaking.
- degeneration of myelin in the spinal cord in multiple sclerosis;
- infection, e.g. acquired immunodeficiency syndrome (AIDS);
- diseases involving the vertebral column and/or intervertebral discs, e.g. ankylosing spondylitis and prolapsed intervertebral discs.
Figure 11.4 Anterolateral (spinothalamic) pathway seen in a frontal section of the brain with spinal cord, and its position in the transverse section of the spinal cord.
Interpretation of pain
In the past, pain was thought to be a unidimensional sensory experience. There was a belief that neural mechanisms responsible for the transmission of pain were solely ‘hard-wired’, and sensory nerves carried ‘damage information’ to a single pain centre in the brain. This single pathway reproduced a pain sensation in proportion to the original damage. In other words, the more damage done, the more pain was felt, and when the damage ‘healed ’, the pain would stop. The return of function would automatically follow. This account of the experience of pain leads to frustration when individuals still report pain, in the absence of ongoing tissue damage, and it is inferred that the pain the person reports is imaginary.
It is now well recognised, especially by people who experience pain, that tissue healing after damage does not always stop pain. The current view of neural mechanisms responsible for the transmission of pain is that they have a dynamic/plastic nature with the capacity to change. The formulation and continuation of pain is a multidimensional experience that incorporates sensory, emotional, affective, cognitive and behavioural elements. The interaction between people and the environment is also affected. Individuals may report spontaneous ongoing pain, pain during occupations that would not be expected to provoke it, and chronic pain over many years despite the absence of tissue damage. In other words, the extent and severity of pain appear to be disproportionate to the original damaging stimulus. This is because pain is a perception that is the sum of many individual mechanisms occurring in the central nervous system and therefore is reported to be multidimensional in nature.
In summary, there is no single route from nociceptors to the somatosensory cortex or other areas of the brain that can explain how pain is experienced at any one time. Pain is a subjective perception and different individuals may interpret the same damaging (noxious) stimulus in different ways at different times.
In order to understand more fully why this is the case, the different types of pain that individuals can perceive must be appreciated.
Transient pain is usually brief in duration and is of little consequence, because tissue damage is minimal. Accidentally sticking a pin into the finger, would be an example of this type of pain. The sensation is usually sharp and then a dull sensation is experienced, which usually subsides quickly. A function of this type of pain is to prevent further damage by initiating escape from the stimulus and protection of the body.
Acute pain describes pain of recent onset and is probably time limited. It is usually associated with disease or injury that takes longer for the body to repair than transient pain. Acute pain that lasts for more than 3 months may be classified as chronic.
Chronic pain lasts for long periods, for example years, and persists beyond tissue healing. This may occur in chronic conditions such as joint disease, nerve damage or cancer. However, chronic pain may also be experienced in the absence of tissue damage. It is now thought that pain mechanisms and neural pathways can become dysfunctional and undergo plastic changes leading to maladaptive responses. Chronic pain by definition is more than a sensation and is multidimensional in nature.
Neural mechanisms of transient and acute pain
Peripheral and central mechanisms have been studied to understand the perception of pain. Once understood, the plastic changes that can take place in the nervous system contributing to the perception of chronic pain can be appreciated.
The mechanisms in the ‘normal state ’ of transient and acute pain are presented as transduction, transmission, perception and modulation.
Transduction : this is the process of converting the energy content of a stimulus applied to a receptor into action potentials in sensory nerve fibres. Nociceptors are activated by noxious stimuli, which may be mechanical, thermal or chemical (released from damaged tissues), or any combination. The energy of the stimulus is converted into electrochemical impulses in the sensory neurones with small -diameter nerve fibres transmitting noxious information from the periphery to the spinal cord (see Chapter 1 ). Nociceptors are normally only activated transiently by intense levels of stimulation. Long-term stimulation by locally released endogenous pain-producing chemicals associated with tissue damage can result in changes in receptor sensitivity. If the receptors become more sensitive (hyperalgesia), the experience of pain is exaggerated. Pain may also be felt from a stimulus that would not normally cause pain (allodynia).
Remember there are other types of receptor in the skin, joints and muscles responding to non -noxious tactile and proprioceptive stimuli. When activated, this information istransmitted towards the spinal cord in large -diameter fibres.
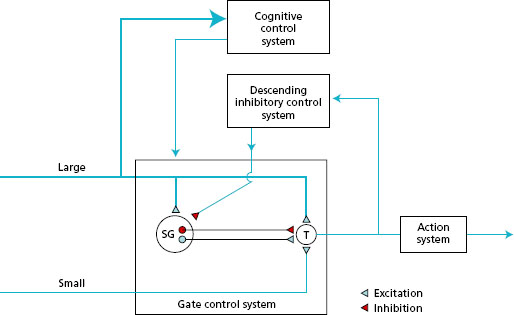
Transmission : noxious information in small-diameter fibres and non -noxious information in large -diameter fibres is transmitted to the spinal cord and directed to the posterior horns. In the posterior horn there is a layer known as the substantia gelatinosa (SG cells), owing to its appearance, composed of interneurones with short axons. The sensory neurones synapse with the SG cells and in turn with the transmission (T) cells, the fibres of which link with the ascending pathways to the brain. This pain gate mechanism in the spinal cord integrates the incoming information so that onward transmission of information towards the cortex depends on the balance between noxious and non -noxious input. Figure 11.5 shows the SG and T cells in the gate control system, which will be considered further under modulation.
The ascending pathways include the spinothalamic tract (STT) and the spinoreticular tract (SRT).
- The STT is a direct nociceptive pathway that ascends in the cord, synapses in the thalamus and then goes on to the somatosensory strip of the cortex (see Figure 11.4 ).
- The SRT is a less direct nociceptive, multiple pathway that ascends the cord and synapses in the medial aspect of the thalamus and then to multiple regions of the brain.
Perception: pain is not perceived as ‘pain’ until it is interpreted within various structures and areas of the cerebral cortex. This implies that pain is not merely a sensation but a perceptual experience. Nociceptive information via the STT is projected from the thalamus to the primary somatosensory cortex, where the pure sensory component of pain is registered and discriminated in terms of quality, intensity, localisation and duration. The STT is responsible for the primary processing of pain, which centres on identification of stimuli. Some information is also transmitted to the brain stem, where the reticular formation influences the state of arousal of the cortex.
Nociceptive information via the SRT is projected diffusely and bilaterally to many other areas of the brain. This secondary processing of pain, related to recognition and meaning, is dependent on the association areas of the brain that form part of the cognitive control system (Figure 11.5). The prefrontal lobe initiates the cognitive evaluation of pain and the development of coping strategies. The hypothalamus and the limbic system are involved in the development of pain behaviours. The hypothalamus regulates autonomic responses, e.g. blood pressure and breathing; and the limbic system is concerned with mood and emotional responses. The individual may exhibit holding and guarding a limb, adopting awkward postures, facial grimacing and wincing, vocalising pain, and feeling nauseous, sweaty and light-headed. The construction of the perception of pain relates both to volition and mood (motivational-affective components) and to the future implications of pain in the individual’s life (cognitive-evaluative components).
Figure 11.5 Gate control theory based on Melzack & Wall (1983). SG = cells in substantia gelatinosa; T = transmission cells. (Redrawn from Main C.J. & Spanswick C.C. (2000) Pain Management-An Interdisciplinary Approach, Churchill Livingstone, Edinburgh, with kind permission.)