CHAPTER 80 Sacral Fractures
Anatomic and Biomechanical Considerations
The sacrum is the lowest functional portion of the spinal column and provides an anchor for the mobile lumbar spine. Lumbosacral motion occurs through the lumbosacral intervertebral disc and the paired zygapophyseal (facet) joints. In addition to the usual intervertebral stabilizing structures, the lumbosacral articulation is further stabilized by the iliolumbar ligaments connecting the L5 transverse processes to the crest of the ilium and the sacrolumbar ligaments that have an origin contiguous with the iliolumbar ligament and insert into the anterosuperior aspect of the sacrum and sacroiliac joint.1 These structures make the L5-S1 motion segment inherently more stable than the more cephalad lumbar motion segments.
The sacrum has an inclination that is balanced by a cascade of lumbar lordosis, thoracic kyphosis, and cervical lordosis so that a plumb line from C7 typically passes through the posterior aspect of the L5-S1 intervertebral disc. The sacral inclination angle, defined as the angle between a tangent to the dorsal surface of the sacrum and a vertical reference line, varies between 10 and 90 degrees and is usually between 45 and 60 degrees.2,3 Pelvic incidence measures the orientation of the lumbosacral junction relative to the pelvis and approximates 50 degrees in the general population.4 It is normally referred to in the context of sagittal plane malalignment in lumbar spondylolisthesis but is also valuable in determining sagittal alignment when treating sacral fractures. Sacral fractures that significantly alter spinal sagittal alignment, usually by shifting the C7 plumb line anteriorly, may result in difficulty with maintaining an erect posture, compensatory hyperlordosis, and associated functional deficits.5
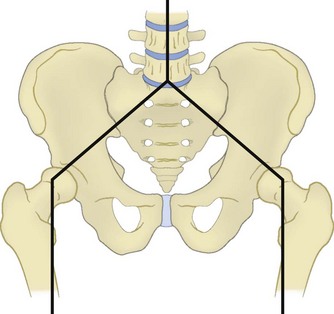
The sacrum forms the posterior segment of the pelvic ring through the sacroiliac joints. Forces transmitted through the lumbosacral articulation into the upper sacrum propagate laterally across the sacroiliac joints to the pelvis (Fig. 80–1). Because of these load-transferring properties, the sacrum has been described as the keystone of the pelvic ring.6 More specifically, the sacrum functions as a “true keystone” in the pelvic outlet plane, in which the orientation of the sacrum relative to the ilium is such that axial forces lock the sacrum into the pelvic ring and further stabilize the sacroiliac articulation. In the pelvic inlet plane the sacrum is shaped like a “reverse keystone” and its position in this plane is inherently unstable, permitting pelvic ring motion (Fig. 80–2). This pelvic ring instability necessitates substantial intrinsic and extrinsic ligamentous stabilization of the sacroiliac joints. Intrinsic stability is provided by the substantial interosseous ligaments and posterior sacroiliac ligaments, as well as the relatively weaker anterior sacroiliac ligaments. Extrinsic stability is provided by the sacrospinous and sacrotuberous ligaments in the pelvic floor.7
The sacrum itself is composed of five kyphotically aligned vertebral segments. The upper two sacral segments are similar in size to the lumbar vertebrae, whereas the lower three sacral segments rapidly diminish in size.8 Intersegmental fusion begins between the lowest two segments around the age of 15 and proceeds in a cephalad direction until the sacrum is completely fused by 25 to 30 years of age. Significant variability in upper sacral anatomy can exist in the form of transitional vertebrae and sacral dysplasia. Forms of transitional vertebrae include a sacralized lumbar segment, an incomplete congenital fusion of L5 to the sacrum that is usually unilateral, and a lumbarized sacrum that denotes a persistent articulation between the S1 and S2 segments.9 Complete or bilateral sacralization may result in a dysplastic sacral morphology with the upper sacrum located cephalad relative to the iliac crests. Because upper sacral variability results in significant alteration in the relationships among the sacrum, pelvis, and spinal column, as well as their adjacent neurovascular structures, it must be recognized during the evaluation and treatment of sacral fractures, particularly if surgical treatment is considered.10
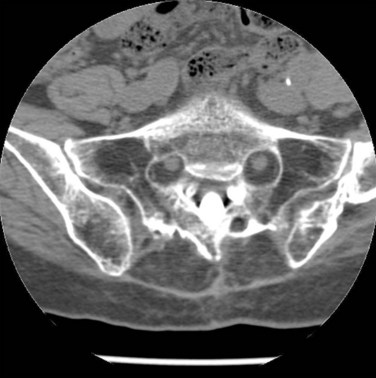
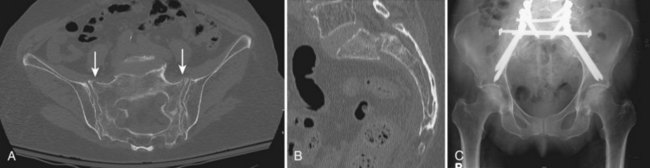
The upper sacral body, termed the base of the sacrum, consistently has the highest density of cancellous bone, with the S1 segment adjacent to the superior endplate having the greatest density overall. The ventral aspect of the upper S1 body that projects anteriorly and superiorly into the pelvis is termed the sacral promontory. The sacral ala, the lateral portion of the sacrum that articulates with the ilium through the sacroiliac joints, is largely cancellous and is formed by the coalescence of the sacral transverse processes. There is wide variability in sacral alar morphology among individuals, and the ala can be a substantial broad shoulder of bone or a narrow, partially segmented structure. The cancellous alar bone is hypodense, particularly in older individuals, and an alar void is a consistent finding in middle-aged and older adults (Fig. 80–3).11,12 The relative difference in bone density between the upper and lower sacral body predisposes this area to fracture. The hypodense ala is predisposed, particularly in the older and osteopenic person, to fracture line propagation. This effect is accentuated by the relative strength of the sacroiliac joint ligaments that stabilize and prevent their displacement or disruption. Similarly, younger individuals injured before complete ossification of the sacrum are predisposed to disruption at the S1-2 level owing to relative weakness at that interval. The relatively sparse alar bone density must be taken into consideration when planning reconstructive procedures.
The posterior surface of the sacrum is convex and is formed by the coalescence of posterior elements. Accordingly, the middle sacral crest corresponds to the spinous processes, the intermediate sacral crests correspond to the zygapophyseal joints, and the area in between corresponds to the laminae. The lowest one or two sacral segments have incompletely formed bony posterior elements, resulting in an aperture into the sacral spinal canal: the sacral hiatus. Enlargement of the sacral hiatus may relatively weaken the sacrum and predispose it to fracture.13
Sacral pedicles and laminae form the lateral and posterior borders of the spinal canal as it tapers in size distally from a triangular cross section at the S1 level to a flat and narrow cross section at the most caudal segments. The dural sac typically ends at the S2 level, and the filum terminale attaches its caudal end to the coccyx. At the junction of the body and the sacral ala are four paired ventral and dorsal neuroforamina through which the sacral nerve root ventral and dorsal rami pass, respectively. The relative space available to the sacral nerve roots in the ventral foramina is lowest at the S1 and S2 levels, where the nerve roots occupy one third to one fourth of the foraminal space compared with the S3 and S4 levels, where the nerve roots occupy one sixth of the available foraminal space. It follows that the lower sacral roots are less likely to be impinged upon in injuries involving displacement of the neuroforamina.14
Numerous structures are adjacent to the sacrum, which may be injured in sacral fracture-dislocations. The majority are neurovascular structures, with the rectum being the only visceral structure in close proximity. Below the rectosigmoid junction, located at the S3 level, the mesentery disappears and the colon lies directly adjacent to the sacrum.15
The dorsal nerve roots exit through their respective posterior neuroforamina to supply motor branches to the paraspinous muscles and cutaneous sensory branches that form the cluneal nerves. Anteriorly, the L5 nerve root is intimately associated with the sacrum as it passes underneath the inferior edge of the sacrolumbar ligament and drapes over the anterosuperior aspect of the sacral ala. It anastomoses with the L4 ventral ramus and in passing caudad adjacent to the sacral ala is joined by the exiting ventral sacral nerve roots to form the sacral plexus.16 Branches of the sacral plexus include the sciatic nerve, pudendal nerve, superior gluteal nerve, and inferior gluteal nerve. In addition, the sympathetic chain lies directly on the ventral sacrum immediately medial to the neuroforamina. At each level it distributes a gray ramus communicans to the ventral nerve root. Therefore the sacral plexus mediates major lower extremity function, as well as bowel, bladder, and sexual function. The dual innervation of the perineal structures from both the left and right sacral plexus is protective of bowel, bladder, and sexual function. These functions are largely preserved in the event of transection of the sacral nerve roots unilaterally, whereas bilateral transection causes complete loss of function.17
The presacral area has an extensive vascular network that is highly variable. The middle sacral artery typically courses ventrally along the midline of the L5 vertebral body, across the sacral promontory, and down the sacrum after branching from the aorta at the common iliac bifurcation. The common iliac arteries subsequently give rise to the internal iliac arteries that lie anterior to the sacroiliac joints and give off both superior and inferior lateral sacral arteries. The superior lateral sacral artery traverses the upper sacroiliac joint and proceeds caudad just lateral to the sacral foramina, giving off spinal arteries that pass through the S1 and S2 ventral foramina to the spinal canal. The inferior lateral sacral artery traverses the inferior aspect of the sacroiliac joint before anastomosing with the middle sacral artery and giving off spinal arteries that pass through the S3 and S4 ventral foramina.9 The internal iliac veins lie posteromedially to the internal iliac arteries and course caudad. They are located medial to the sacroiliac joint directly adjacent to the sacral ala.15 The internal iliac veins are the repository of an extensive presacral venous plexus. This plexus is formed by extensive anastomoses between the lateral and middle sacral veins and receives transforaminal veins that communicate with epidural veins in the spinal canal.1,8,18 This extensive vascular network renders anterior exposures to the sacrum impractical and perilous.
History and Classification
Bonnin reported the first classification of sacral fractures in 1945.19 He divided sacral fractures by mechanism of injury into those occurring as a result of either direct or indirect forces. Direct injuries occurred from a projectile or a fall. These were isolated injuries to the sacrum and had minimal or no impact on either spinal or pelvic instability. Conversely, with indirect injuries resulting from the transmission of forces through a disrupted pelvic ring, he described several anatomic patterns of injury including the propensity of the sacrum to fracture through the S1 and S2 neuroforamina at the junction of the ala and the body, resulting in a “broken link in the solid connections between the ilium and the vertebral column.” In addition to pelvic ring and/or spinal column instability, anatomic patterns of injury that included the neuroforamina or spinal canal were noted to be associated with possible neurologic deficit. Specifically, S1 or S2 nerve root injury was common in his cases. Bowel and bladder dysfunction as a result of lower sacral nerve root injury was sometimes also seen.19
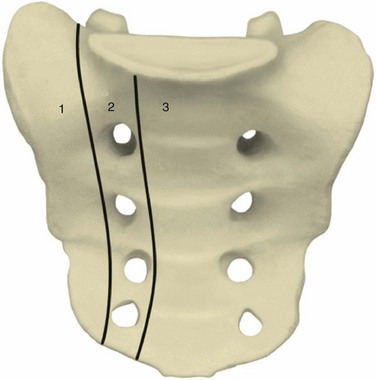
Several other classification schemes based on the identification of additional injury patterns were subsequently proposed.20,21 None was widely adopted until 1988 when Denis and colleagues,14 based on a series of 236 sacral fractures, formulated a simplified anatomic classification that correlates fracture location with the incidence of neurologic injury. This classification divides the sacrum into three zones (Fig. 80–4). Zone I, or alar zone, fractures remain lateral to the neuroforamina throughout their course. Zone II, or foraminal zone, fractures are located in the transition area between the sacral ala and body and involve one or more neuroforamina while remaining lateral to the spinal canal. Zone III, or central zone, fractures involve the spinal canal. The key relationship is that the incidence of neurologic injury increases as fractures are more centrally located. Accordingly, in the series of Denis and colleagues, zone I fractures had a 5.9% incidence of neurologic injury, primarily to the L5 nerve root as it coursed over the ala. Zone II fractures had a 28.4% incidence of associated neurologic injury occurring as a result of either foraminal displacement and resulting impingement on the exiting nerve root or the “traumatic far-out syndrome” in which that L5 nerve root is caught between the L5 transverse process and the displaced sacral ala. Zone III fractures had a 56.7% incidence of neurologic injury resulting from injury at the level of the spinal canal, with 76.1% of these individuals having bowel, bladder, and sexual dysfunction. Denis and colleagues14 noted a spectrum of zone III sacral fracture-dislocations including both transverse and longitudinal fracture line orientations and described a subtype that they termed a sacral burst fracture consisting of retropulsion of the sacral body with an intact sacral lamina.
Gibbons and colleagues22 subsequently considered 44 cases of sacral fracture according to the Denis classification system. They found a 34% incidence of neurologic injury overall. Six of 25 patients (24%) with zone I injuries had L5 and/or S1 nerve root injuries. Two of seven patients with zone II injuries similarly had L5 and S1 deficits. No patient with zone I or II injuries had bowel or bladder dysfunction. Three of five patients with zone III injuries had neurologic injury, two with bladder dysfunction. Gibbons and colleagues22 used these findings as a basis for a classification of neurologic injury from sacral fractures. Neurologic injuries were classified as 1, none; 2, paresthesias only; 3, motor loss with bowel and bladder intact; and 4, bowel and/or bladder dysfunction. They also noted that significant neurologic deficit is rare in transverse sacral fractures below the S4 level.
Huittinen,23 in a postmortem study of 42 individuals with posterior pelvic ring disruptions, studied the incidence and anatomic basis of nerve injuries. A total of 40 lumbosacral nerve injuries were identified in 20 of the autopsies. Fifty-three percent were traction injuries and 38% were ruptures. Compression injuries, the most amenable to surgical intervention, were present in only 20% of the nerve injuries.
Because of the high potential for neurologic injury, as well as spinal column instability, sacral body (Denis zone III) fractures have been specifically considered by several investigators. In 1969 Purser reported a case of transverse fracture of the sacrum across the S1-2 interval with bilateral upper sacroiliac joint disruption and L5 transverse process avulsions.24 There was anterior angulation without significant translation of the upper sacrum. Although the fracture passed through the S1 neuroforamina, there was minimal foraminal displacement and the patient was neurologically intact. Subsequently, in 1976 Bucknill and Blackburne25 reported three cases of transverse fractures of the upper sacrum all associated with neurologic deficits. All three patients had lower extremity weakness, and one had bladder dysfunction. Weaver26 and colleagues described transverse sacral fractures as analogous to the traumatic L5-S1 spondylolisthesis described by Nicoll in 194927 that resulted from a flexion moment applied in a position of hip flexion and knee extension. Schmidek and colleagues agreed that transverse sacral fractures are a variation of traumatic L5-S1 spondylolisthesis that he attributed to the relative bony weakness in the upper sacrum, particularly in skeletally immature patients before ossification is complete.21 Fountain and colleagues28 further differentiated upper transverse sacral fractures from lower transverse sacral fractures that occur from a direct fall resulting in the coccyx levering the sacrum and fracturing it at the S3 level, the apex of the sacrum, and the caudal end of the sacroiliac joint. They reported a series of six patients who had three upper and three lower sacral fractures. All six initially had neurologic dysfunction from cauda equina injury manifesting as urinary retention, decreased anal sphincter tone, or both. There have been several other case reports of transverse fractures of the sacrum. Review of these case reports is remarkable for a high association of neurologic deficit characterized as cauda equina injury affecting lower extremity, as well as bowel and bladder function.20,26,29–37
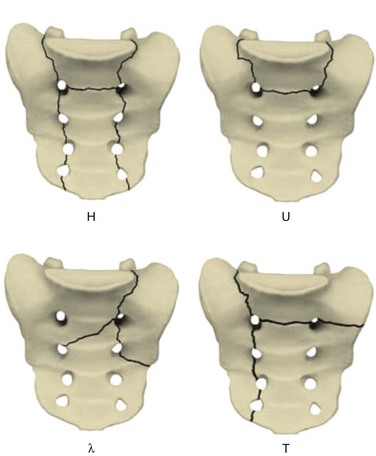
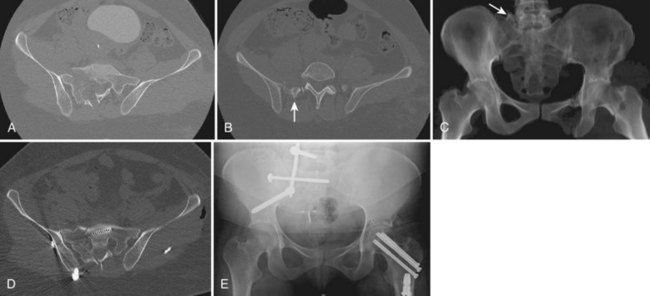
Early case reports often characterized the injury pattern as solely a transverse fracture, possibly owing to imaging limitations. Computed tomography (CT) demonstrates that most transverse fractures of the upper sacrum have complex fracture patterns. The majority of these injuries are now understood to consist of a transverse fracture of the sacrum with associated longitudinal or “vertical” injury components, usually in the form of bilateral transforaminal fractures that extend rostrad to the lumbosacral junction, the so-called “U” fracture. Variations in fracture line propagation include the “H,” “Y,” and “lambda” fracture patterns (Fig. 80–5). There is also a high incidence of L5 transverse process fractures, indicating disruption of the iliolumbar ligament.33
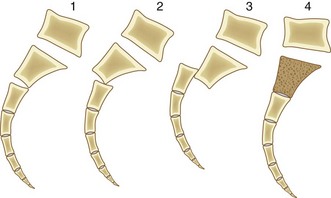
Roy-Camille and colleagues38 reviewed their experience of 13 patients with transverse sacral fractures, 11 as a result of attempted suicide by jumping. They classified the fractures as type I, flexion deformity of upper sacrum (angulation alone); type II, flexion deformity with posterior displacement of the upper sacrum on the lower sacrum (angulation and posterior translation); and type III, anterior displacement of the upper sacrum without angulation (anterior translation alone). On the basis of cadaveric studies, they hypothesized that types I and II were caused by impact with the lumbar spine in flexion, whereas type III fractures were caused by impact with the lumbar spine and hips in extension.38 Strange-Vognsen and Lebech39 subsequently reported a case of comminution of the upper sacrum without significant angulation or translation that they termed a type IV injury relative to the Roy-Camille classification. They hypothesized that in type IV injuries the lumbar spine was in the neutral position at the point of impact (Fig. 80–6).
Other specific patterns of sacral fractures have been identified as resulting from specific mechanisms or having predictable patterns of associated injuries. In particular, in contrast to transverse Denis zone III sacral fractures, midline longitudinal Denis zone III sacral fractures have a low incidence of neurologic injury (Fig. 80–7).40–42 This injury, in which the sacrum is disrupted through a sagittal plane, was originally reported by Wiesel and colleagues in 1979, who suggested that these fractures may have a lower incidence of neurologic injury than transverse fractures because the nerve roots are displaced laterally instead of transected.42 Bellabarba and colleagues40 subsequently described this injury as a variant of the anteroposterior compression pelvic ring injury. In their series of 10 patients, none had neurologic deficits, in contradistinction to the high incidence of neurologic injury reported in patients with predominantly transverse sacral fractures through the spinal canal.40
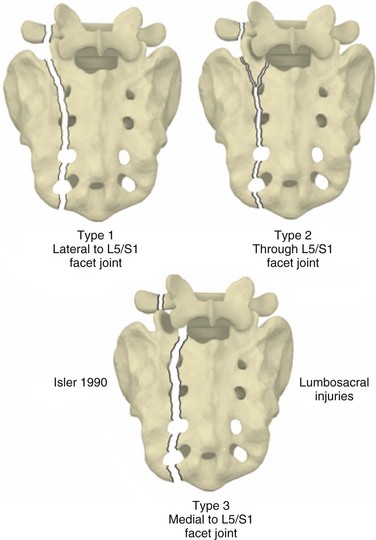
Even in the absence of a transverse fracture line, sacral fractures can be associated with spinal column instability. Isler described variations of longitudinal sacral fractures through the S1 and S2 neuroforamina that result in L5-S1 motion segment instability owing to facet joint disruption (Fig. 80–8).43 Injuries with the fracture line lateral to the S1 articular process are not associated with instability of the lumbosacral articulation because the L5-S1 articulation remains continuous with the stable fracture component of the sacrum. Fracture that extends into or medial to the S1 articular process, however, may disrupt the associated facet joint and potentially destabilize the lumbosacral junction (Fig. 80–9). Complete displacement of the facet joint can cause a locked facet joint, making sacral fracture reduction difficult with closed methods alone. Facet disruption may also cause post-traumatic arthrosis and late lumbosacral pain.
In contrast to sacral fractures that occur as a result of high-energy mechanisms of injury, the precipitating event in sacral insufficiency fractures is often not identifiable or may be related to a fall onto the buttocks from a standing or sitting position. Insufficiency fractures are stress fractures that occur in weakened bone, and these injuries typically occur in postmenopausal women due to osteoporosis. Conditions contributing to the osteoporosis such as chronic corticosteroid use or a history of radiation therapy to the pelvis are often present (Fig. 80–10).27,44 The presenting symptoms are often vague and frequently consist of poorly localized low back pain that may be exacerbated by sitting and standing. These fractures are typically oriented vertically and occur through the ala adjacent to the sacroiliac joint. There may also be a transverse component resulting in more complex “U”-fracture variants. Because of the predisposition of these patients to osteoporotic fractures, there is high association with insufficiency fractures at other sites, most commonly the pubis and thoracolumbar vertebrae. Neurologic deficits are uncommon in most series.45,46 Cauda equina dysfunction has been reported, however, and neurologic status must be carefully considered.47
Mechanical factors related to the transfer of forces from the lumbosacral spine to the pelvis may also cause sacral insufficiency fractures in the patient predisposed to osteoporosis.39 Specifically, unilateral sacral insufficiency fractures have been identified contralateral to the convex side of lumbar scoliosis.45 Pelvic stress concentration as the result of lumbosacral spine fusion has also been described,48 and sacral insufficiency fractures caudal to previous lumbar fusions are also increasingly being reported. These fractures probably represent failure of the sacrum to withstand forces concentrated there as a result of the large cephalad lever arm.49–53
Evaluation
Sacral fractures therefore occur in two distinctly different patient groups. Most commonly, sacral fractures are the result of high-energy trauma. As noted previously, they are also increasingly being seen in patients with osteopenic bone disorders that predispose to pathologic fractures. In both groups of patients, diagnosis is frequently delayed, which may result in further displacement or neurologic deterioration. In their retrospective analysis of 236 patients with sacral fractures, Denis and colleagues14 found that in the neurologically intact patient the diagnosis of sacral fractures was made during the initial hospitalization only 51% of the time. The presence of a neurologic deficit increased the diagnostic accuracy to only 70%. As in most cases of diagnostic delay, the etiology of missed sacral fractures is multifactorial and ranges from the relative difficulty in identifying these fractures on screening anteroposterior pelvis radiographs combined with the presence of distracting injuries in the trauma patient to low clinical suspicion in patients with insufficiency fractures.14,54
In the trauma patient the transfer of energy resulting in a fracture of the sacrum often causes other injuries including life-threatening head and thoracoabdominal trauma. In these patients emergent resuscitation is the top priority. Accordingly, Advanced Trauma Life Support (ATLS) protocol begins with a primary survey during which conditions that are immediately life threatening are addressed. Resuscitation is focused on maintaining cardiopulmonary and hemodynamic stability. Only after this primary goal has been achieved should the secondary survey, composed of examination of the patient to identify additional injuries, be completed.55
The secondary survey includes screening evaluation of both the spinal column and pelvic ring. Precautions to maintain spinal column integrity are necessary, and patients should be initially maintained on a flat surface and log-rolled side to side to prevent spinal column displacement. Evaluation includes inspection and palpation of the patient’s back from the occiput to the coccyx. Significant findings associated with sacral fractures are the presence of skin discoloration or laceration, palpable stepoff or crepitus, and ballotable fluid in the lumbopelvic region. Significant soft tissue contusion or internal degloving such as a Morel-Lavallee lesion can have important implications on subsequent treatment.56 Rectal and vaginal examination including the use of a speculum and proctoscope allows for detection of occult open sacral fractures.
Because pelvic ring disruption may be associated with significant intrapelvic hemorrhage, provisional methods of pelvic ring stabilization may be necessary to reduce pelvic volume and provide provisional stability. These methods include the application of a circumferential pelvic antishock sheet,10 pelvic clamp, anterior external fixator, or skeletal traction depending on the pelvic ring fracture pattern. Associated vascular injury, particularly to the hypogastric arterial system, may require embolization to adequately control arterial hemorrhage.57
Determining the patient’s neurologic status is of paramount importance in patients with sacral fractures.58–60 A rectal examination is performed early in the workup of all multiply injured patients, even in the absence of obvious sensorimotor deficits in the extremities, to evaluate perianal sensation, anal sphincter tone, and voluntary perianal contraction and to assess for presence of anal wink and the bulbocavernosus reflex. A straight-leg raise test should also be performed in the cognitively unimpaired patient to assess for entrapment of the lumbosacral plexus. Extremity motor function is graded on a scale of 0 to 5 according to the American Spinal Injury Association (ASIA) modification of the Frankel grading system, and a sensory level is obtained. Determination of level of injury by neurologic examination is limited to the L5 and S1 levels, however, and injuries to the lower sacral roots cannot be more specifically identified than obtaining a perianal sensory level.
Fracture displacement can cause neurologic injury from a variety of mechanisms including angulation, translation, and direct compression by displaced bone fragments. Potentially reversible injuries include contusion, compression, and traction. Recovery of transected or avulsed nerve roots cannot be expected. Delayed neurologic deficit can occur from epidural hematoma, late fracture displacement, or callus formation19 and should be promptly reinvestigated to determine its cause.
There are multiple adjunctive methods for the evaluation of the neurologic status in a patient with a sacral fracture. Electrodiagnostic studies are particularly valuable in the evaluation of cognitively impaired patients and are also useful in differentiating upper motor neuron injuries or spinal cord injury from cauda equina injury in patients with head or proximal spinal column injury, in the evaluation of patients with urinary tract injury, and for intraoperative monitoring.61 Conventional electromyography (EMG) and somatosensory-evoked potentials (SSEPs) are useful in the evaluation of L5 and S1 function. Pudendal SSEPs and anal sphincter EMG are necessary for evaluation of the sacral roots below the S1 level.62–65 Possible abnormalities on sphincter EMG include detrusor areflexia, uninhibited sphincter relaxation, or denervation. EMG is limited in the acute setting because these abnormalities may take weeks to emerge. For patients with neurogenic bladder, serial postvoid residuals or cystometrography is a useful diagnostic aid. In patients with elevated postvoid residuals, other causes such as impaired function due to narcotic inhibition must be considered.
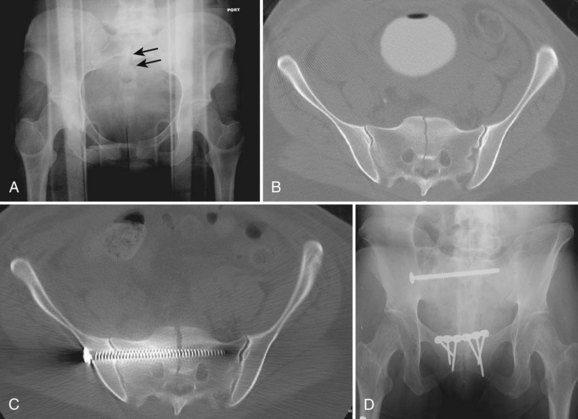
The advent of more sophisticated imaging techniques in the routine initial assessment of the trauma patient’s visceral injuries such as CT of the abdomen and pelvis has assisted the detection of previously unrecognized sacral fractures. The identification of a sacral fracture on these studies mandates complete radiographic evaluation including a dedicated CT scan of the sacrum with 2-mm or less axial cuts and sagittal and coronal re-formations to allow the detail required for determining the fracture configuration, resulting instability pattern, and extent of sacral canal and neuroforaminal compromise (Fig. 80–11).66 Three-dimensionally reformatted CT scans may add insight into fracture morphology for less experienced clinicians or in the case of highly complex fracture configurations. Magnetic resonance imaging (MRI) is not usually helpful except in cases of unclear neurologic deficits or discrepancies between skeletal and neurologic levels of injury, although it may provide early evidence of lumbosacral nerve root avulsion.67 Recent advances in MRI neurography allowing for visualization of the lumbosacral plexus injuries may be of some help in their evaluation, although at this point it is neither a reliably effective nor a practical diagnostic tool and more clinical experience with this modality is necessary.
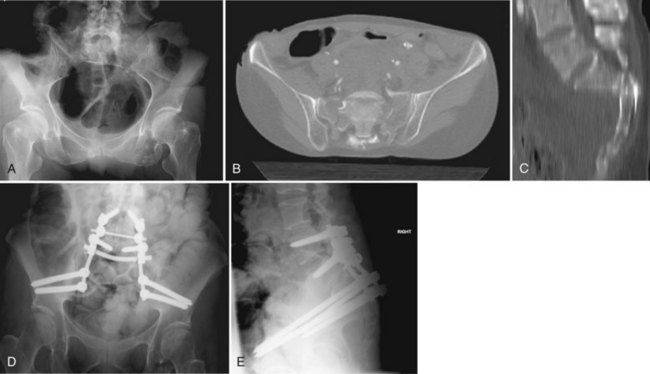
FIGURE 80–11 A to C, Sacral U fracture in suicidal jumper. D and E, After treatment with lumbopelvic fixation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree