CHAPTER 17 Rotator Cuff
HISTORICAL REVIEW
It is often difficult to tell where concepts actually begin. It is certainly not obvious who first used the term rotator cuff or musculotendinous cuff. Credit for first describing ruptures of this structure is often given to J. G. Smith, who in 1834 described the occurrence of tendon ruptures after shoulder injury in the London Medical Gazette.1 In 1924, Meyer published his attrition theory of cuff rupture.2 In his 1934 classic monograph, Codman summarized his 25 years of observations on the musculotendinous cuff and its components and discussed rupture of the supraspinatus tendon.3 Beginning 10 years after the publication of Codman’s book and for the next 20 years, McLaughlin wrote on the etiology of cuff tears and their management.4,5 Arthrography, with air used as the contrast medium, was first carried out by Oberholtzer in 1933.6 Lindblom and Palmer7 used radiopaque contrast and described partial-thickness, full-thickness, and massive tears of the cuff.
Codman recommended early operative repair for complete cuff tears and carried out what may have been the first cuff repair in 1909.8 Current views of cuff tear pathogenesis, diagnosis, and treatment are quite similar to those that he proposed.
Pettersson has provided an excellent summary of the early history of published observations on subacromial pathology. Because of its completeness, his account is quoted here.9
The first to observe morbid processes in the subacromial bursa was Jarjavay,10 who on the basis of a few cases gave a general description of subacromial bursitis. His views were modified and elaborated by Heineke11 and Vogt.12 Duplay13 introduced the term “periarthritis humeroscapularis” to designate a disease picture characterized by stiffness and pain in the shoulder joint following a trauma. Duplay based his observations on cases of trauma to the shoulder joint and on other cases of stiffness in the shoulder following dislocation, which he had studied at autopsy. The pathological foundation for the disease was believed by Duplay to lie in the subacromial and subdeltoid bursa. He thought that the cause was probably destruction or fusion of the bursa.
Duplay’s views, which were supported by his followers Tillaux14 and Desché,15 were hotly disputed. His opponents, Gosselin and his pupil Duronea16 and Desplats,17 Pingaud and Charvot,18 tried to prove that the periarthritis should be regarded as a rheumatic affection, neuritis, etc.
In Germany, Colley19 and Küster20 were of practically the same opinion regarding periarthritis humeroscapularis as Duplay. Roentgenography soon began to contribute to the problem of humeroscapular periarthritis. It was not long before calcium shadows began to be observed in the soft parts between the acromion and the greater tuberosity.21 The same finding was made by Stieda,22 who assumed that these calcium masses were situated in the wall and in the lumen of the subacromial bursa. These new findings were indiscriminately termed “bursitis calcarea subacromialis” or “subdeltoidea.” The term “bursoliths” was even used by Haudek23 and Holzknecht.24 Later, however, as the condition showed a strong resemblance to humeroscapular periarthritis, it became entirely identified with the latter.
In America, Codman25 made a very important contribution to the question when he drew attention to the important role played by changes in the supraspinatus in the clinical picture of subacromial bursitis. Codman was the first to point out that many cases of inability to abduct the arm are due to incomplete or complete ruptures of the supraspinatus tendon.
With Codman’s findings it was proved that humeroscapular periarthritis was not only a disease condition localized in the subacromial bursa, but that pathological changes also occurred in the tendon aponeurosis of the shoulder joint. This theory was further supported by Wrede,26 who, on the basis of one surgical case and several cases in which roentgenograms had revealed calcium shadows in the region of the greater tuberosity, was able to show that the calcium deposits were localized in the supraspinatus tendon.
More and more disease conditions in the region of the shoulder joint have gradually been distinguished and separated from the general concept, periarthritis humeroscapularis. For example, Sievers27 drew attention to the fact that arthritis deformans in the acromioclavicular joint may give a clinical picture reminiscent of periarthritis humeroscapularis. Bettman28 and Meyer and Kessler29 pointed to the occurrence of deforming changes in the intertubercular sulcus, the canal in which the biceps tendon glides. Payr30 attempted to isolate the clinical picture which appears when the shoulder joint without any previous trauma is immobilized too long in an unsuitable position. Julliard31 demonstrated apophysitis in the coracoid process (coracoiditis) as forming a special subdivision of periarthritis. Wellisch32 described apophysitis at the insertion of the deltoid muscle on the humerus, giving it the name of “deltoidalgia.” Schár and Zweifel33 described deforming changes in connection with certain cases of os acromiale.
The cuff story continues with recognition of subacromial abrasion as a possible element in rotator cuff disease by a number of well-known surgeons, including Codman,25 Armstrong,34 Hammond,35,36 McLaughlin,4 Moseley,37 Smith-Petersen and colleagues,38 and Watson-Jones.39 Some of these surgeons proposed complete acro-mionectomy for relief of these symptoms,34–36,39,40 whereas others advocated lateral acromionectomy.4,38 The term impingement syndrome was popularized by Charles Neer in 1972.41 In 100 dissected scapulae, Neer found 11 with a
characteristic ridge of proliferative spurs and excrescences on the undersurface of the anterior process (of the acromion), apparently caused by repeated impingement of the rotator cuff and the humeral head, with traction of the coracoacromial ligament…. Without exception it was the anterior lip and undersurface of the anterior third that was involved.41
It is surely of relevance that the spurs were thought to be the result of impingement rather than its cause. Neer emphasized that the supraspinatus insertion to the greater tuberosity and the bicipital groove lie anterior to the coracoacromial arch with the shoulder in the neutral position and that with forward flexion of the shoulder, these structures must pass beneath the arch, thereby providing the opportunity for abrasion. He suggested a continuum from chronic bursitis and partial tears to complete tears of the supraspinatus tendon that can extend to involve rupture of other parts of the cuff. He pointed out that the physical examination and plain radiographic findings were not reliable in differentiating chronic bursitis and partial tears from complete tears. Importantly, he emphasized that patients with partial tears seemed more prone to increased shoulder stiffness and that surgery in this situation was inadvisable until the stiffness had resolved. He described the use of a subacromial lidocaine injection to help localize the clinical problem and before acromioplasty as a “useful guide of what the procedure would accomplish.”41
Neer described three different stages of the impingement syndrome:
He emphasized the importance of nonoperative management of cuff tendinitis. If surgery was performed, Neer pointed out the importance of preserving a secure acromial origin of the deltoid, performing a smooth resection of the undersurface of the anteroinferior acromion, making a careful inspection for other sources of abrasion (e.g., the undersurface of the acromioclavicular joint) and ensuring careful postoperative rehabilitation.41–43
In 1972, Neer41 described the indications for acromioplasty as complete tears of the supraspinatus or long-term disability from chronic bursitis and partial tears of the supraspinatus tendon. He pointed out that the physical and roentgenographic findings in these two categories were indistinguishable and included crepitus and tenderness over the supraspinatus, with a painful arc of active elevation from 70 to 120 degrees and pain at the anterior edge of the acromion on forced elevation. Neer’s 1983 report42 described candidates for acromioplasty as patients with an arthrographically demonstrated cuff tear; patients older than 40 years with negative arthrograms but persistent disability for 1 year despite adequate conservative treatment (including efforts to eliminate stiffness), provided that the pain can be temporarily eliminated by the subacromial injection of lidocaine; certain patients younger than 40 years with refractory stage 2 impingement lesions; and patients undergoing other procedures for conditions in which impingement is likely (such as total shoulder replacement in those with rheumatoid arthritis or old fractures).
The proposed goal of acromioplasty was to relieve mechanical wear at the critical area of the rotator cuff. Surgery was not considered until any stiffness had resolved and the disability had persisted for at least 9 months. Even in patients with continuing symptoms after lateral acromionectomy, Neer considered anterior acromioplasty because he found that many still had problems related to subacromial impingement. Neer also reported that the rare patient with an irreparable tear in the rotator cuff could be made more comfortable and could gain surprising function if the impingement were relieved, as long as the deltoid origin was preserved.42
Neer42 recommended resection of small unfused acromial growth centers and internal fixation of larger unfused segments in a manner that tilted the acromion upward to avoid impingement. His indications for resection of the lateral aspect of the clavicle included arthritis of the acromioclavicular joint, a need for greater exposure of the supraspinatus in a cuff repair, and nonarthritic enlargement of the acromioclavicular joint, resulting in impingement on the supraspinatus (in this situation, only the undersurface of the joint was resected).42
Bernstein44 had the following to offer regarding impingement:
The term impingement syndrome was first used by Neer in 197241 to describe a condition of shoulder pain associated with chronic bursitis and partial tearing of the rotator cuff. Impingement comes from a Latin root impingo, which means “to strike against.” Neer explicitly stated that in this pathological state, the anterior acromion was striking against (and damaging) the underlying rotator cuff. He described the anterior acromion as “the rough surface on which the supraspinatus tendon is rubbing.”
Neer further described the lateral acromion as “an innocent part,” and recommended removing only the anterior edge. But what if all of the acromion is innocent? McCallister and colleagues45 showed good results of rotator cuff repair performed without acromioplasty, and Budoff and coauthors46 noted that débridement of partial-thickness tears of the rotator cuff without acromioplasty is clinically beneficial as well.
In his commentary on Neer’s article, Zarins stated:
Additional approaches to subacromial abrasion have been proposed, including section of the coracoacromial ligament,47–50 resection arthroplasty of the acromioclavicular joint,51 extensive acromionectomy,40,34–40,52 and combined procedures such as acromioplasty, incision of the coracoacromial ligament, acromioclavicular resection arthroplasty, and excision of the intra-articular portion of the biceps tendon with tenodesis of the distal portion of the bicipital groove.53–55
Comparison of the results of these procedures is difficult because of the heterogeneous patient groups and varying methods of evaluation. In 16 patients with chronic bursitis and fraying or a partial tear of the supraspinatus, Neer41 found that 15 attained satisfactory results (no significant pain, less than 20 degrees of limitation in overhead extension, and at least 75% of normal strength). Thorling and coworkers56 found good to excellent results in 33 of 51 patients after acromioplasty (in 11, resection of the acromioclavicular joint was performed as well).
More recently, arthroscopic acromioplasty has been introduced. The frequency with which this procedure is performed has increased dramatically as the strictness of Neer’s original indications for acromioplasty has been allowed to relax. Ellman57 presented the initial results of 50 consecutive cases of arthroscopic acromioplasty for stage II impingement without a cuff tear (40 cases) and for full-thickness cuff tears (10 cases). Eighty-eight percent of the patients had excellent or good results, and the rest had unsatisfactory results at a 1- to 3-year follow-up. He pointed out that the technique was technically demanding. Difficulties with arthroscopic acromioplasty range from inadequate subacromial smoothing on the one hand to transection of the acromion or virtually total acromionectomy on the other. In his early series of 100 arthroscopic acromioplasties, Gartsman58 found that at an average of 18.5 months’ follow-up, 85 shoulders were improved and 15 were failures; 9 of the failures required subsequent open acromioplasty. The procedure took longer than open acromioplasty and did not speed the patient’s return to work or sports. A comparison between open and arthroscopic acromioplasties by Spangehl and colleagues showed no significant differences in outcome based on University of California at Los Angeles (UCLA) scores and patient satisfaction. Patients who had open acromioplasty had significantly more pain relief at a minimum of 1 year of follow-up.59 Morrison60 reported a series of arthroscopic acromioplasties in which the quality of the result was closely correlated with conversion of a curved or hooked acromion to a flat undersurface.
RELEVANT ANATOMY AND MECHANICS
The Skin
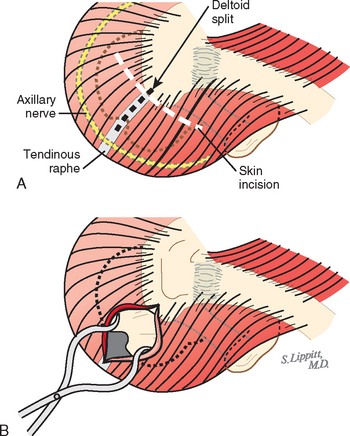
Rotator cuff surgery can usually be accomplished through cosmetically acceptable incisions in the lines of the skin (Fig. 17-1). These skin lines run obliquely in a superolateral-to-inferomedial direction. The usual superior approach to the cuff is made through an oblique incision that runs over the anterior corner of the acromion. In most cases, this incision heals with a scar less noticeable than those resulting from arthroscopic portals.
Deltoid Muscle
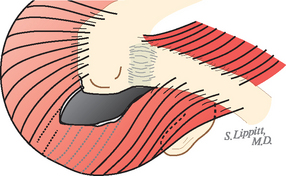
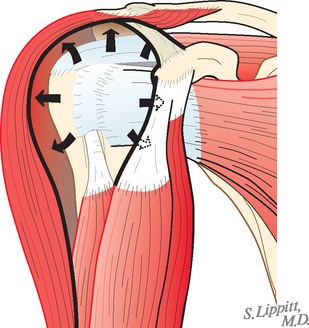
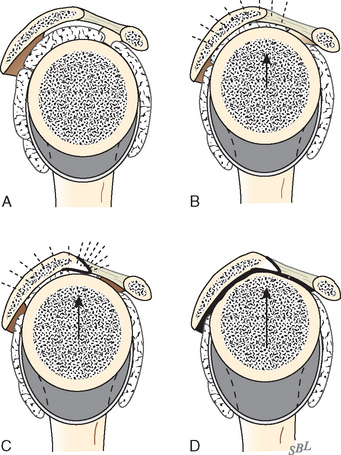
The deltoid arises from the lateral half of the clavicle, the acromion, and posteriorly from the scapular spine. Because the origin of the deltoid is over the entire anterior height of the acromion, a substantial amount of the deltoid origin must be detached when performing an acromioplasty, whether the procedure is carried out by open means or arthroscopically (Figs. 17-2 and 17-3).61 The deltoid has an important and constant tendon of origin separating its anterior and lateral thirds. This tendon attaches to the anterolateral corner of the acromion, in which location it provides the key to the anterior deltoid-splitting approach to the cuff. By making the deltoid split down the center of the tendon, the surgeon can be assured of having strong tendinous handles on the muscle to use in closing the deltoid (see Fig. 17-1). Although it is often said that the location of the axillary nerve is on average 5 cm distal to the acromion, its anterior branches swoop upward, so it is desirable to limit the inferior extent of the deltoid split to minimize the risk of injury to the axillary nerve branches (see Fig. 17-1).
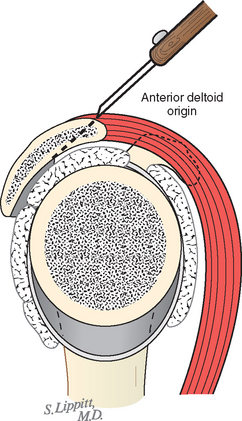
FIGURE 17-2 Loss of the deltoid origin. Acromioplasty sacrifices part of the deltoid origin.
(From Matsen FA III, Lippitt SB: Shoulder Surgery: Principles and Procedures. Philadelphia: WB Saunders, 2004, pp 391, 266.)
Scapula, Acromion, Coracoid, and Coracoacromial Ligament
The fact that the scapula can be moved on the chest wall enables the scapulohumeral muscles to be used at their best advantage (Figs. 17-4 and 17-5).
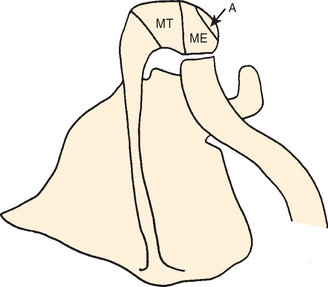
The acromion is a scapular process arising from three separate centers of ossification: a pre-acromion, a meso-acromion, and a meta-acromion.62–64 These centers of ossification are usually united by 22 years of age. When these centers fail to unite, the ununited portion is referred to as an os acromiale (Fig. 17-6). This condition might have first been recognized by Schár and Zweifel33 in 1936, as was mentioned by Pettersson.9 Grant65 found that 16 of 194 cadavers older than 30 years demonstrated incomplete fusion of the acromion; the condition was bilateral in 5 subjects and unilateral in 11. In a large review of 1000 radiographs, Liberson66 found unfused acromia in 2.7%; of these, 62% were bilateral. Most commonly the lesion is a failure of fusion of the mesoacromion to the meta-acromion. He found the axillary view to be most helpful in revealing the condition. The size of the unfused fragment may be substantial, up to 5 × 2 cm.41 Resection of a fragment this large creates a serious challenge for deltoid reattachment.
Norris and coworkers67 and Bigliani and associates68 pointed to an association of cuff degeneration and an unfused acromial epiphysis. Mudge and coworkers63 found that 6% of 145 shoulders with cuff tears had an os acromiale, whereas Liberson found a 2.7% incidence of this finding in unselected scapulae.66 The statistical and clinical significance of this association remains unclear. Warner and associates studied 14 patients with os acromiale who were treated by open reduction and internal fixation. Overall, 50% achieved union, but 86% achieved union with a tension band and cannulated screw fixation versus 14% with a tension band alone. Of patients who achieved union, 86% had good results, but only 14% of patients with nonunion were considered to have a good result.69 Boehm and colleagues looked at 62 patients with an os acromiale in the axillary radiographs of 1000 patients undergoing rotator cuff repair. The average number of tendons and average age was similar between those with and without the os acromiale. They concluded that the prevalence of os acromiale in patients with tears is comparable to that in a standard population with an unknown status of the rotator cuff. Thus, it seemed debatable to them whether an os acromiale is a pathologic condition leading to rotator cuff tears.70
The coracoid arises from two or three ossification centers.64 It provides the medial attachment site for both the coracohumeral and coracoacromial ligaments. In that their muscle bellies lie medial to it, the neighboring supraspinatus and subscapularis tendons must be able to glide by the coracoid with their full excursion during shoulder movement. Scarring of one or both of these tendons to the coracoid can inhibit passive and active shoulder motion. Although the coracoid does not normally contact the anterior subscapularis tendon, forced internal rotation, particularly in the presence of a tight posterior capsule, can produce such contact as a result of the obligate translation.71,72
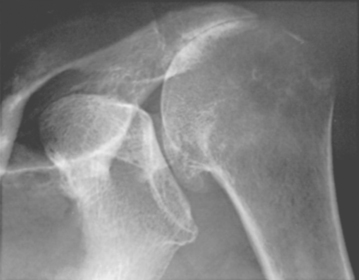
The coracoacromial ligament spans from the undersurface of the acromion to the lateral aspect of the coracoid and is continuous with the less-dense clavipectoral fascia. It forms a substantial part of the superficial aspect of the humeroscapular motion interface (Figs. 17-7 and 17-8). This ligament may be thought of as the spring ligament of the shoulder in that it maintains the normal relationships between the coracoid and the acromion. Separation of these two scapular processes has been observed on sectioning this ligament in cadavers.73 Calcification in the acromial end of this ligament can give the appearance of a subacromial spur (Fig. 17-9). An important study by Chambler and coworkers discussed their findings in acromial spurs of 15 patients undergoing rotator cuff repair. They found high levels of activity of ALP (alkaline phosphatase) and G6PD (glucose-6-phosphate dehydrogenase), both indicating osteoblast activity, at the acromial insertion of the coracoacromial ligament to provide evidence of bone remodeling. They considered this further evidence that spur formation was a secondary phenomenon in the presence of established rotator cuff tears.74
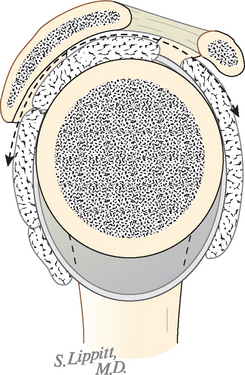
The coracoacromial arch is the inferiorly concave smooth surface consisting of the anterior undersurface of the acromion and the coracoacromial ligament. It provides a strong ceiling for the shoulder joint, along which the cuff tendons must glide during all shoulder movements (see Fig. 17-8). Passage of the cuff tendons and the proximal end of the humerus under this arch is facilitated by the subacromial–subdeltoid bursa, which is not normally a space, as often shown on diagrams, but rather two serosal surfaces in contact with each other, one on the undersurface of the coracoacromial arch and deltoid and the other on the cuff. These sliding surfaces are lubricated by the bursal surfaces and synovial fluid.
Recognition of the gliding articulation between the arch and the cuff is not new. Renoux and colleagues credited Ludkewitch, who in 1900 recognized that proper functioning of the “scapulohumeral articulation” requires the presence of a “secondary socket” that extends the glenoid fossa of the scapula above, in front, and behind, with the coracoacromial arch forming the ceiling.75 In 1934, Codman stated that the coracoacromial arch was an auxiliary joint of the shoulder and that its roughly hemispheric shape was “almost a counterpart in the size and curvature of the articular surface of the true joint.” He referred to the “gleno-coraco-acromial socket.”3 His belief in the importance of the coracoacromial arch was great enough to state that “the coracoacromial ligament has an important duty and should not be thoughtlessly divided at any operation.” Wiley has pointed to the severe superior instability that results when the ceiling of the shoulder is lost in association with cuff deficiency.76 Kernwein and associates in 1961 stressed the importance of the “suprahumeral gliding mechanism” consisting of the coracoacromial arch on one side and the rotator cuff and biceps tendon on the other separated by the subacromial bursa.77 They believed that these two opposing, gliding surfaces and interposed bursa constituted a fifth joint that contributed to shoulder motion. DePalma, in 1967, also recognized the intimate relationship between the arch and the structures below it.78 He referred to the arch, together with the head of the humerus, the rotator cuff, and the subacromial bursa, as the “superior humeral articulation.”
Matsen and colleagues described the humeroscapular motion interface as an articulation (see Figs. 17-7 and 17-8) between the cuff, the humeral head, and the biceps on the inside and the coracoacromial arch, the deltoid, and the coracoid muscles on the outside79; they measured up to 4 cm of gliding at this articulation in normal shoulders in vivo.
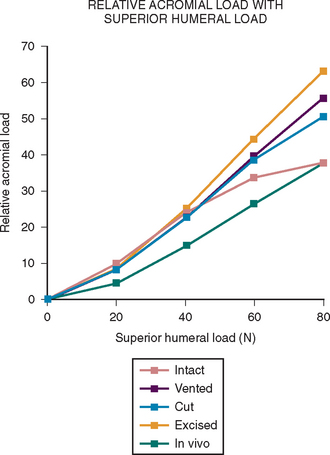
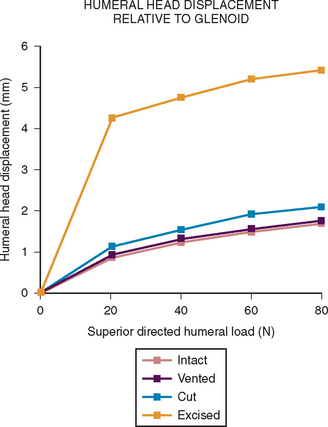
More recent investigations73,80–84 have pointed to the importance of contact and load transfer between the rotator cuff and the coracoacromial arch in the function of normal shoulders, including the provision of superior stability. Because there is normally no gap between the superior cuff and the coracoacromial arch, the slightest amount of superior translation compresses the cuff tendon between the humeral head and the arch. Superior displacement is opposed by a countervailing force exerted by the coracoacromial arch through the cuff tendon to the humeral head. Ziegler and collaborators84 demonstrated this “passive resistance” effect in cadavers by showing that the acromion bent upward when a superiorly directed force was applied to the humerus in the neutral position. The amount of acromial deformation was directly related to the amount of superior force applied to the humerus, the load being transmitted through the intact superior cuff tendon. Furthermore, these authors found that the amount of superior humeral displacement resulting from a superiorly directed humeral load of 80 N was increased from 1.7 to 5.4 mm when the cuff tendon was excised (P < .0001) (Figs. 17-10 and 17-11). These results indicate that an intact superior cuff tendon is subject to compressive loading between the humeral head and the coracoacromial arch and that the presence of this tendon provides passive resistance against superior displacement of the humeral head when superiorly directed loads are applied.
Flatow and coauthors73 also noted in a dynamic cadaver model that the presence of the supraspinatus tendon limited superior translation of the humeral head, even when no tension was placed on the tendon from simulated muscle action.
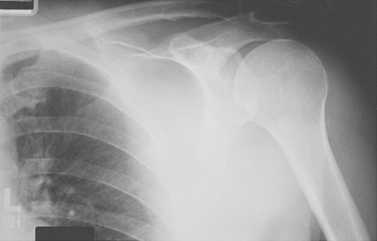
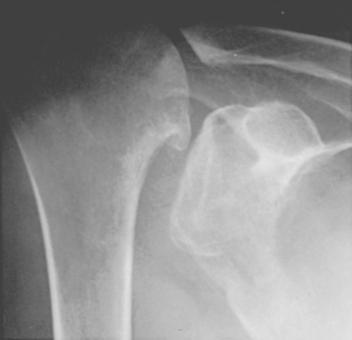
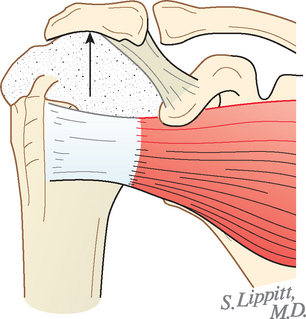
The spacer effect of the superior cuff tendon is evident when comparing shoulders with intact cuffs (Fig. 17-12) with those in which the superior tendon is deficient (Fig. 17-13).
FIGURE 17-13 Loss of the passive resistance effect. The acromiohumeral interval of the right shoulder is narrowed because of loss of the interposed superior cuff tendon in the same patient as in Figure 17-12.
Ziegler and colleagues and Flatow and coworkers cautioned that superior stability of the shoulder depends on an intact coracoacromial arch. Surgical sacrifice of the arch can lead to severe superior instability (Figs. 17-14 and 17-15).
Changes in the coracoacromial arch have been described in association with cuff disease85 along with variations in acromial shape.41,68 From 2% to 4% of asymptomatic college athletes with an average age of 19.9 years were found to have type III acromial morphology, whereas 12% to 14% and 84% to 85% had type I and II acromial morphology, respectively.86 Another study of asymptomatic persons showed no correlation of acromial morphology with age, but acromial and clavicular spurring was significantly associated with increasing age in persons without shoulder symptoms.87
Bigliani and colleagues88 studied 140 shoulders in 71 cadavers. The average age was 74.4 years. They identified three acromial shapes: type I (flat) in 17%, type II (curved) in 43%, and type III (hooked) in 40%. Fifty-eight percent of the cadavers had the same type of acromion on each side. Thirty-three percent of the shoulders had full-thickness tears, 73% of which were seen in association with type III acromia, 24% with type II, and 3% with type I. The anterior slope of the acromion in shoulders with cuff tears averaged 29 degrees, slightly more than the slope of those without cuff tears, which averaged 23 degrees. The clinical significance of this relatively small difference is not known.
A number of other authors have reported that patients with cuff defects are more likely to have hooked or angled acromia.89–91 Nicholson and coworkers92 demonstrated in a review of 420 scapulae that spur formation on the anterior acromion was an age-related process such that persons younger than 50 years had less than one fourth the prevalence of those older than 50 years. The status of the cuffs of these shoulders is unknown. A study by Gill and associates93 showed that in patients older than 50 years, no significant association between acromial morphology and rotator cuff pathology could be found. However, there was a significant correlation with age and rotator cuff tears. Older patients were more likely to have type II and III acromia, although this study did not comment on whether this trend was significant. These findings imply that apparent correlations between acromial morphology and rotator cuff tears may be exaggerated because of the confounding variable of age. A study of contact geometry between the undersurface of the coracoacromial arch and the rotator cuff revealed no significant differences in cadaveric shoulders with and without rotator cuff tears.94
A rotator cuff tear subsequently developed in 20% of patients who underwent an acromioplasty, thus suggesting that acromioplasty does not prevent the occurrence of rotator cuff tears.95 In a remarkable study, Ozaki and colleagues96 correlated the histology of the acromial undersurface with the status of the rotator cuff in 200 cadaver shoulders. Cuff tears that did not extend to the bursal surface were associated with normal acromial histology, whereas those that extended to the bursal surface were associated with pathologic changes in the acromial undersurface. They concluded that most cuff tears are related to tendon degeneration and that acromial changes are secondary to pathology of the bursal side of the cuff. These results are similar to those reported Fukuda and coauthors.97 The contact geometry of the rotator cuff and the undersurface of the coracoacromial arch was not significantly different between cadaveric shoulders with and without rotator cuff tears.94
Other studies suggest that type II and III acromia are acquired rather than developmental.98 In that most acromial hooks lie within the coracoacromial ligament (Figs. 17-16 and 17-17), it seems likely that they are actually traction spurs in this ligament (analogous to the traction spur seen in the plantar ligament at its attachment to the calcaneus) (Figs. 17-18 and 17-19, see Fig. 17-9). The traction loads producing this hook can result from loading of the arch by the cuff and may be increased with increasing dependency on the coracoacromial arch for superior stability in the presence of cuff degeneration.73,81,84
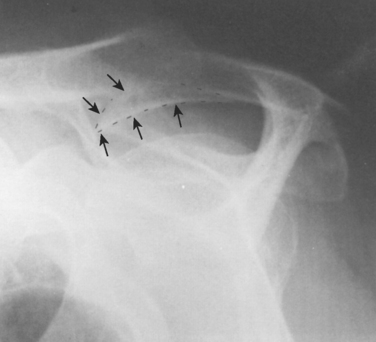
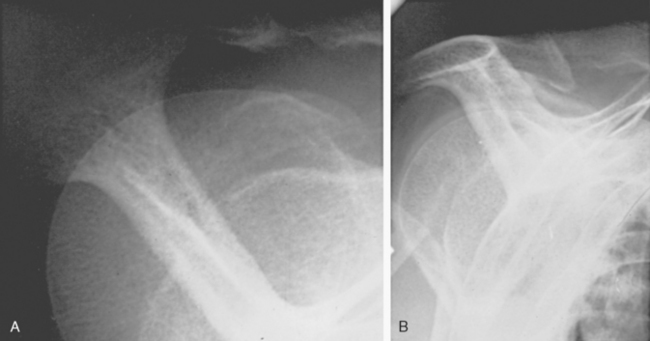
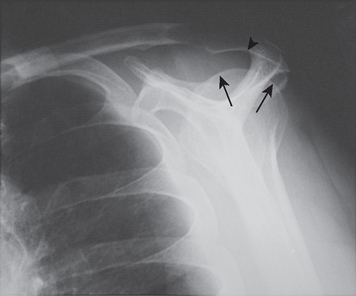
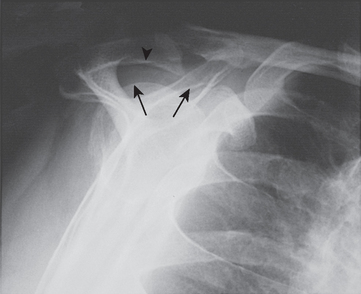
FIGURE 17-17 Supraspinatus outlet radiograph demonstrating a large anterior inferior osteophyte (arrows).
(From Iannotti JP: Rotator Cuff Disorders: Evaluation and Treatment. Rosemont, Ill: American Academy of Orthopaedic Surgeons, 1991.)
The concept of the hook as a traction phenomenon was first put forward by Neer more than 30 years ago.41 More recently, Putz and Reichelt99 reported that three quarters of 133 operative specimens of the coracoacromial ligament showed chondroid metaplasia near the acromial insertion, thus suggesting that this metaplastic area becomes the acromial hook by enchondral bone formation.100 Because this hook lies within the ligament and points toward the coracoid (see Fig. 17-17), it seems unlikely that it would jeopardize passage of the cuff beneath the coracoacromial arch (see Fig. 17-18). Even in the severest cases of cuff tear arthropathy, the undersurface of the coracoacromial arch commonly presents a smooth articulating concavity (Fig. 17-20, see Fig. 17-18). The smoothness of the undersurface of the coracoacromial arch can be verified by palpation at surgery, even in cases in which an acromial spur is suggested by radiographs. Ogawa and colleagues found that small spurs were associated with advancing age and that spurs measuring 5 mm or more were associated with bursal-sided tears and full-thickness tears.101
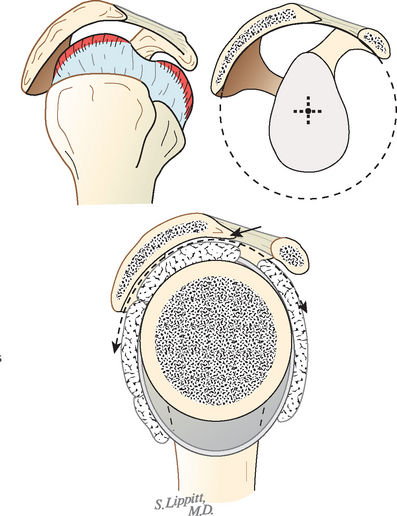
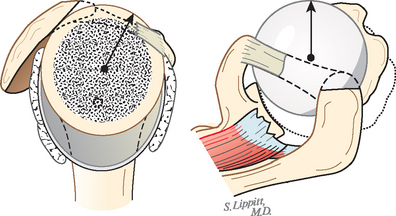
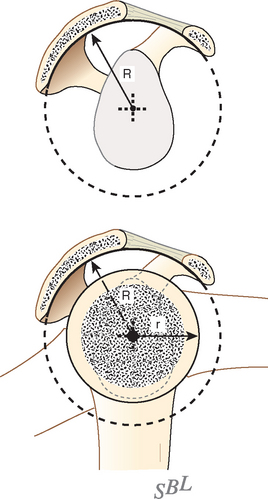
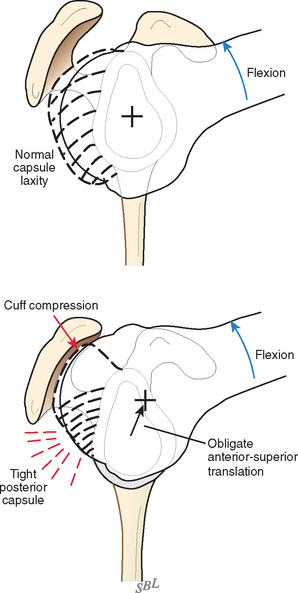
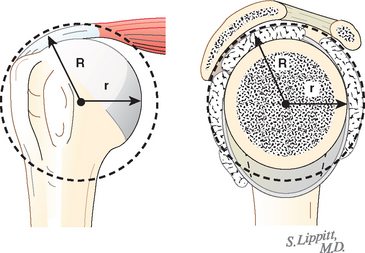
In view of the foregoing, it is instructive to consider the humeroscapular articulation as consisting of two concentric spheres, the glenohumeral sphere and the sphere represented by the articulation between the inferior surface of the coracoacromial arch and the proximal humeral convexity. Together, these two spheres enhance both shoulder stability and the surface available for scapulohumeral load transfer (Fig. 17-21).3 Normally, the spheres of the humeral head and the coracoacromial arch share the same center. The difference in radius of the two spheres (R and r in Fig. 17-21) is provided by the thickness of the rotator cuff, which serves as a spacer. In the presence of posterior capsular tightness, shoulder flexion or internal rotation (or both) causes obligate anterosuperior translation of the humeral head and loss of the concentricity of the two spheres (Fig. 17-22).72,102 As a result, the proximal humeral convexity is forced against the anterior undersurface of the concave coracoacromial arch rather than rotating concentrically beneath it (see Figs. 17-21 and 17-22). In the presence of degeneration of the cuff tendon, the shoulder can lose the concentricity of the humeral head and coracoacromial arch spheres (Figs. 17-23 to 17-25). Taken together, these observations reinforce the shoulder’s need for normal posterior capsular laxity; a smooth, concentric, and congruent coracoacromial undersurface; and a normally thick and uniform cuff interposed between the humeral head and the coracoacromial arch.
Acromioclavicular Joint
Theoretically, osteophytes from the acromioclavicular joint may encroach on the space normally occupied by the cuff tendons (Fig. 17-26). However, these spurs are usually too medial to contact the tendons. In a series of 47 patients with arthrographically confirmed supraspinatus tendon rupture, Peterson and Gentz103 found that 51% had distally pointing acromioclavicular joint osteophytes. A similar incidence was found in their series of 170 autopsy specimens with cuff defects. The incidence of distally pointing acromioclavicular osteophytes in normal shoulders was 14% in the clinical studies and 10% in the cadaver studies. It is recognized, however, that degenerative changes in the cuff and degenerative changes in the acromioclavicular joint can coexist in the aging population without the former being causally related to the latter. Steinbeck and colleagues pointed out in their retrospective study of 102 patients in whom cuff repair was performed with resection of the lateral end of the clavicle in 20, that the results were not significantly influenced by the clavicle resection.104 Again, it is recognized that acromioclavicular osteophytes are usually sufficiently medial that they do not jeopardize the cuff.
Rotator Cuff
Anatomy
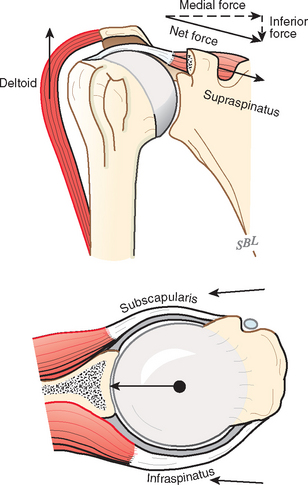
The rotator cuff is a complex of four muscles that arise from the scapula and whose tendons blend in with the subjacent capsule as they attach to the tuberosities of the humerus. The subscapularis arises from the anterior aspect of the scapula and attaches over much of the lesser tuberosity. It is innervated by the upper and lower subscapular nerves.105 The supraspinatus muscle arises from the supraspinatus fossa of the posterior portion of the scapula, passes beneath the acromion and the acromioclavicular joint, and attaches to the superior aspect of the greater tuberosity. It is innervated by the suprascapular nerve after it passes through the suprascapular notch. The infraspinatus muscle arises from the infraspinous fossa of the posterior portion of the scapula and attaches to the posterolateral aspect of the greater tuberosity. It is innervated by the suprascapular nerve after it passes through the spinoglenoid notch. The teres minor arises from the lower lateral aspect of the scapula and attaches to the lower portion of the greater tuberosity. It is innervated by a branch of the axillary nerve.
The cross-sectional area of the anterior belly of the supraspinatus was found to be larger than its posterior belly. The tendon of the anterior muscle belly was found to have a smaller cross-sectional area than that of its posterior belly, however.106 This increase in muscle strength–to–tendon area ratio might explain why rotator cuff tears often begin in the anterior portion of the supraspinatus. The superior portion of the subscapularis was found to be stiffer than its inferior portion.107
Bey and associates108 found that the strain within the rotator cuff increased in positions of greater abduction, even when the same force was applied through the rotator cuff. No significant difference in strain on the superficial and deep portions of the cuff was detected. The compressive stiffness of the articular side of the supraspinatus tendon was found to be significantly less than that of its bursal side.109 This difference in stiffness might be one reason that most tears begin on the articular surface.
For an excellent review of the anatomy and histology of the rotator cuff, the reader is referred to the works of Clark and Harryman110–112 and Warner’s chapter on shoulder anatomy in The Shoulder: A Balance of Mobility and Stability.113
The long head of the biceps tendon may be considered a functional part of the rotator cuff. It attaches to the supraglenoid tubercle of the scapula, runs between the subscapularis and the supraspinatus, exits the shoulder through the bicipital groove under the transverse humeral ligament, and attaches to its muscle in the proximal part of the arm. Slätis and Aalto114 point out that the coracohumeral ligament and the transverse humeral ligament keep the biceps tendon aligned in the groove. Tension in the long head of the biceps can help compress the humeral head into the glenoid. This tendon also has the potential for guiding the head of the humerus as it is elevated, with the bicipital groove traveling on the biceps tendon like a monorail on its track. This mechanism helps explain why the humerus is capable of substantial rotation when it is adducted and allows very little rotation when it is maximally abducted (in which position the tuberosities are constrained as they straddle the biceps tendon near its attachment to the supraglenoid tubercle).
Mechanics
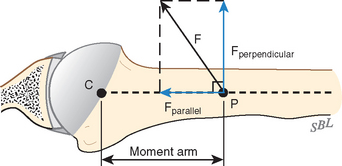
The mechanics of cuff action is complex. The humeral torque resulting from contraction of a cuff muscle is determined by the moment arm (the distance between the effective point of application of this force and the center of the humeral head) and the component of the muscle force that is perpendicular to it (Fig. 17-27).115
The magnitude of force that can be delivered by a cuff muscle is determined by its size, health, and condition, as well as the position of the joint. The cuff muscles’ contribution to shoulder strength has been evaluated by Colachis and associates,116,117 who used selective nerve blocks and found that the supraspinatus and infraspinatus provide 45% of abduction and 90% of external rotation strength. Howell and coworkers118 measured the torque produced by the supraspinatus and deltoid muscles in forward flexion and elevation. They found that the supraspinatus and deltoid muscles are equally responsible for producing torque about the shoulder joint in the functional planes of motion. Generation of force in abduction of the arm decreased only 5% after a simulated tear of one third and two thirds of the supraspinatus. Detachment of the entire tendon resulted in a 17% loss of strength. Simulation of retraction of tears of one third, two thirds, and the entire supraspinatus resulted in losses of 19%, 36%, and 58%, respectively. These losses were almost completely regained with side-to-side repair.119 Other estimates of the relative contributions of the rotator cuff to shoulder strength have been published.88,120,121
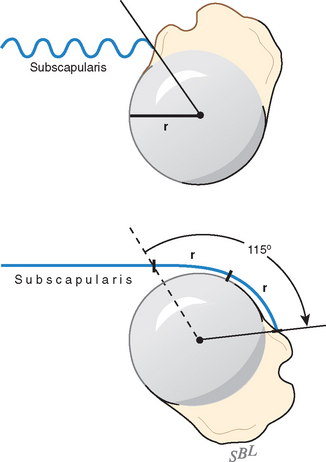
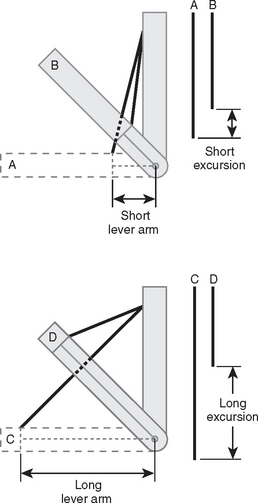
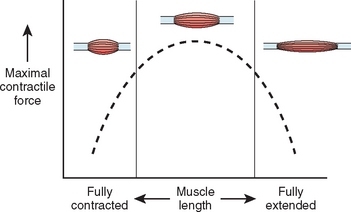
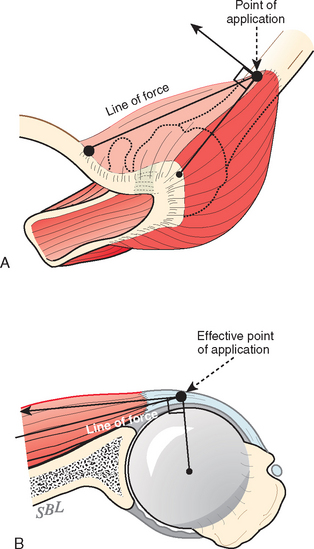
At least three factors complicate analysis of the contribution of a given muscle to shoulder strength. The force and torque that a muscle can generate vary with the position of the joint: Muscles are usually stronger near the middle of their excursion and weaker at the extremes (Figs. 17-28 to 17-30).122 The direction of a given muscle force is determined by the position of the joint. For example, the supraspinatus can contribute to abduction or external rotation, or both, depending on the initial position of the arm.123 The effective humeral point of application for a cuff tendon wrapping around the humeral head is not its anatomic insertion but rather at the point where the tendon first contacts the head, a point that usually lies on the articular surface (Fig. 17-31).
The cuff muscles may be thought of as having three functions. They rotate the humerus with respect to the scapula. They compress the head into the glenoid fossa, thereby providing a critical stabilizing mechanism to the shoulder known as concavity compression. Although in the past the cuff muscles were referred to as head depressors, it is evident that the inferiorly directed components of cuff muscle force are small; instead, the primary stabilizing function of the cuff muscles is through head compression into the glenoid (Fig. 17-32).124,125 They provide muscular balance, a critical function that must be discussed in some detail here.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree