ACUTE MEDICAL AND SURGICAL MANAGEMENT
Clinical practice guidelines (CPG) have recently been published on the early management of adults with SCI (
24). The treatment of a traumatic SCI begins at the scene. An injury to the spinal column should be suspected whenever trauma occurs. As such all trauma victims should have their spine immobilized, preferably with a rigid cervical collar with supportive blocks on a backboard, with straps to secure the entire spine in patients with a potential spinal injury, and should be transferred onto a firm padded surface while maintaining spinal alignment to prevent skin breakdown. Movement should be via logrolling until spinal injury has been ruled out. Traditional cardiopulmonary resuscitation (CPR) methods should be utilized, minimizing trauma to a potentially unstable cervical spine that is utilizing the jaw-thrust maneuver to access the airway. After injury, prompt resuscitation, stabilization of the spine, and avoidance of additional neurologic injury and medical complications are of greatest importance. During the first seconds after SCI, there is release of catecholamines with an initial hypertensive phase. This is rapidly followed by a state of spinal shock, defined as flaccid paralysis and extinction of muscle stretch reflexes below the injury level, although this may not occur in all patients. Ditunno et al. proposed four phases of spinal shock from initial loss of reflex activity to hyperreflexia (
25). Neurogenic shock, as part of the spinal shock syndrome, is a direct result of a reduction in sympathetic activity below the level of injury, consists of hypotension, bradycardia, and hypothermia, and is common in the acute postinjury period. Parasympathetic (PS) activity predominates, especially in persons with injuries at or above the T6 level. Treatment of hypotension involves fluid resuscitation (usually 1 to 2 L) to produce adequate urine output of greater than 30 cc/h. In neurogenic shock, further fluid administration must proceed cautiously, as the patient
is at risk for neurogenic pulmonary edema, and vasopressors are utilized. Maintenance of mean arterial pressure at approximately 85 mm Hg during the first week postinjury has been associated with improved neurological outcomes (
24,
26).
Bradycardia is common in the acute period in cervical spinal level injury and may be treated, if below 40 per minute or if symptomatic, with intravenous (IV) atropine (0.1 to 1 mg), or prevented with atropine given prior to any maneuver that may cause further vagal stimulation (i.e., nasotracheal suctioning). While significant bradycardia typically resolves within 6 weeks, episodes of persistent bradycardia beyond this time may occur in some severe injuries. Some patients may require implantation of a cardiac pacemaker to facilitate safe mobilization (
27).
Respiratory assessment is critical for acute SCI patients, and should include arterial blood gases and measurement of forced vital capacity (VC) as an assessment of respiratory muscle strength (
28). A VC of less than 1 L indicates ventilatory compromise and the patient usually requires assisted ventilation. Serial assessments should be obtained for those with borderline values. A nasogastric tube should be inserted during the initial assessment period to prevent emesis and potential aspiration. A Foley catheter should be inserted with an acutely for urinary drainage and facilitates accurate assessment of urine output and should be left in place until the patient is hemodynamically stable and strict attention to fluid status is no longer required (
24).
Upon presentation to the emergency department, a baseline neurological examination should be performed, maintaining spinal precautions. Imaging studies including x-rays, computed tomography (CT) scan, or MRI should be employed to assess spinal fracture, instability, and/or spinal cord pathology. A standard trauma series includes crosstable laterals and AP views of the cervical and thoracolumbar spine. Because of the incidence of noncontiguous fractures (10% to 40%), once one fracture is identified careful inspection of the rest of the spine is imperative. CT scans often provide improved visualization of the C1 and C7 vertebrae while MRI provides optimal visualization of the neuronal structures. The spine should remain immobilized until an injury has been definitively excluded or the spine is stabilized either surgically or by application of an appropriate orthotic device. In patients with a stiff spine and midline tenderness, the clinician should suspect a fracture (even if plain x-ray is negative), especially in the presence of spondylosis, ankylosing spondylitis, or diffuse interstitial skeletal hyperostosis (DISH) (
24). In cases of cervical dislocations (if patient is cooperative), weights can be applied to Gardner-Wells tongs to achieve cervical distraction and spinal realignment. Application of a halo or surgery will follow. Forty-seven percent of patients with spine trauma and 64% of patients with SCI have concomitant injuries, including head, chest, rib, and long bone fractures (
29). Therefore, a thorough assessment of the total patient is imperative.
Stab wounds and GSWs generally do not produce spinal instability and therefore usually do not require surgical stabilization or orthotic immobilization. Objects that are embedded around the spinal canal (i.e., knife) should be left in place with removal performed in the operating room under direct visualization of the spinal canal. Bullets that pass through the abdominal viscera are treated with broad spectrum antibiotics and tetanus prophylaxis (
30,
31). Bullets do not have to be removed; however, they can be if accessible while performing another surgical procedure.
In many trauma centers in the United States, intravenous (IV) methylprednisolone (MP) is given to adults after an acute SCI. Mechanisms of action for MP include improving blood flow to the spinal cord, preventing lipid peroxidation, free radical scavenger, and having anti-inflammatory function. The National Acute SCI Study (NASCIS) 2 reported that IV MP given within 8 hours of injury (30 mg/kg bolus and 5.4 mg/kg/h for 23 hours) improves neurologic recovery at 6 weeks, 6 months, and 1 year, although functional recovery was not clearly studied (
32). NASCIS 3 reported that if initiated within 3 hours of SCI, MP should be continued for 24 hours, whereas if initiated at 3 to 8 hours after SCI it should be continued for 48 hours (
33). The administration of MP is not extended beyond 8 hours from SCI or in those with penetrating injuries, as they have shown no benefit and their use is associated with a higher incidence of infections (
34,
35). The benefits and safety of utilizing the NASCIS protocol has been questioned, due to the fact that the findings have not been consistently replicated, concerns regarding methodology and analysis, as well as possibly increased morbidity and mortality in persons administered steroids (
36,
37,
38). The neurosurgical guidelines consider the use of high dose MP to be a treatment option rather than a standard (
39), and the CPG states that there is no clinical evidence to definitively recommend the use of any neuroprotective pharmacologic agent, including steroids, in the treatment of acute SCI to improve functional recovery (
24).
Additional recommendations of the CPG include transferring the SCI patient to a specialized center as soon as possible to decrease complications and hospital LOS. Patients with acute SCI, especially high level tetraplegia, should be assessed for evidence of concomitant traumatic brain injury (TBI) (i.e., assessing for loss of consciousness or post-traumatic amnesia [PTA]). Early stabilizations should be considered for extra-spinal fractures. In cases of high-energy injuries, aortic injury should be evaluated. For anesthesia, avoid the use of succinylcholine after the first 48 hours postinjury (potentially fatal hyperkalemic response). While priapism is frequently seen, it is usually self-limited and does not require treatment. Lastly, it is important to maintain normoglycemia in critically ill, mechanically ventilated patients (
24).
Not all SCI is associated with a spinal fracture or dislocation (
24) and may result from forced extreme range of spinal movement without mechanical abnormality. A high index of suspicion for SCIWORA (SCI without radiological abnormality) is important when evaluating adolescents with sports-related neck trauma or victims of child abuse (especially in children who may be suffering from physical abuse) (
24). See
Chapter 59 for pediatric-related SCIWORA.
Spinal Stability and Principles of Spinal Stabilization
White and Panjabi proposed the most widely accepted theory on spinal instability defining it as “the loss of the ability of the spine, under physiologic loads, to maintain its pattern of displacement so that there is no initial or additional neurological deficit, no major deformity, and no incapacitating pain” (
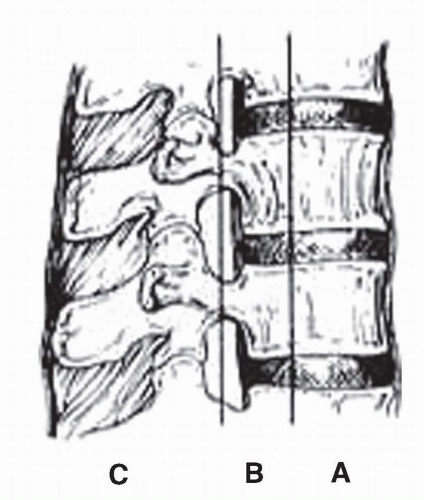
40). This definition is applicable at all levels of the axial spine. Radiographic criteria have been established for the diagnosis of clinical instability of the spine. Denis described the widely accepted “three-column theory” for thoracolumbar fractures, where the spine is divided into three columns (
Fig. 27-1). The anterior column comprises the anterior vertebral body, the anterior longitudinal ligament and the anterior half of the annulus fibrosus. The middle column consists of the posterior vertebral body, the posterior longitudinal ligament, and the posterior half of the annulus fibrosus. The posterior column includes all the posterior elements (including the pedicles). In this three-column theory of Denis, spinal instability is present if any two of the three columns are violated (
41).
Injuries that are primarily ligamentous, such as facet dislocations, are unstable and require internal stabilization procedures (
42). The primary goal of surgical intervention in acute SCI is to decompress the neural elements, and either an anterior or a posterior approach may accomplish this. The approach chosen depends on the expertise of the operating surgeon and the specific pathophysiology of the injury. Since the most common etiology of SCI occurs from retropulsion of bone and/or disc material from a ventral location into the spinal canal, an anterior approach may be preferable. Anterior surgery, however, is associated with increased complications including recurrent laryngeal nerve lesions leading to speech and swallowing disorders. After adequate neural decompression is accomplished, the spine is stabilized and fused. Fusion is typically performed by using autologous bone which is most frequently harvested from the iliac crest. The fibula can also be used as a donor site for autograft bone; however, this is usually reserved for cases that require more than a single level of fusion. Surgical hardware is utilized to help fixate bones in order to allow a fusion to occur. The hardware, however, is only a temporary fixation device that facilitates the eventual long-term bony fusion. From a posterior approach, techniques include use of interspinous wiring with bone grafting or placement of lateral mass plating with bone grafting.
Postoperatively, or if surgery is not required, an orthosis is usually prescribed, and maintained for approximately 3 months. The type of spinal orthotic chosen depends on the level of spinal injury. Generally, for the occipito-C2 levels the Halo-vest may be used, although some surgeons will utilize a head-cervical orthosis (HCO) (i.e., Miami J Collar (Jerome Medical)). An HCO is utilized for the C3-7 levels; for the T1-3 levels a cervicothoracic orthosis is used (i.e., extended HCO or Yale brace). From T4 through L2, a thoracolumbar spinal orthotic (TLSO) is utilized, however at L3 and below a lumbosacral orthotic (LSO) with the incorporation of one hip/thigh (spica attachment to a LSO or TLSO) will ensure satisfactory immobilization of the low lumbar and sacral spine is required.
Specific Injuries to the Spine
Fractures of the atlas are commonly referred to as “Jefferson” burst fractures. These are usually stable injuries (i.e., may occur after a football spearing injury) that may be treated with a Halo-vest orthosis. Unstable Jefferson fractures usually require posterior surgical stabilization. Odontoid fractures are classified into three basic types. Type I odontoid fractures are very rare and involve a fracture of the tip of the odontoid process. Type II odontoid fractures are much more common, particularly in the elderly population, and involve a fracture through the base of the odontoid process, at its junction with the C2 vertebral body. Type III fractures extend from the base of the odontoid into the body of the C2 vertebra proper. Type I odontoid fractures typically require no specific surgical intervention. Type III odontoid fractures are typically treated with an external orthosis (either Halo-vest or HCO) for 3 months. Type II odontoid fractures may be treated with an external halo/vest orthosis, however, there is a high failure rate with this treatment and internal stabilization may be needed.
Fractures of the pedicles of C2 are usually bilateral and are commonly referred to as “hangman’s” fractures. These can occur during an abrupt deceleration injury, that is, a MVC with the person’s head hitting the windshield, and are most often stable injuries treated with external orthoses. When disruption is more significant, treatment with a halo in slight extension
is utilized. In the case of an unstable hangman’s fracture, open surgical fusion may be necessary.
Pure bony injuries in the subaxial spine (C3-7) without substantial neurologic compression may heal with an external orthosis alone. However, most cases of an acute SCI secondary to cervical fractures will have ligamentous injuries and will require open surgical intervention to decompress and/or fuse the cervical spine. The most common burst fracture in the cervical spine occurs at the C5 vertebral level.
The most common thoracic spinal injury involves fracture of the T12 vertebra. Unstable injuries are treated with stabilization and fusion procedures. In the lower thoracic spine, either an anterior or posterior approach may be utilized. Anterior surgery is, typically, performed via a thoracotomy, or a thoracoabdominal approach, when a corpectomy is performed. This is followed by bone grafting and stabilization with either a screw/plate or a screw/rod construct. Anterior surgery in the upper thoracic spine is difficult technically and is rarely performed. Posterior thoracic surgery will classically involve the use of a hook/rod stabilization construct. Stable fractures can be treated with an orthosis (i.e., custom-molded TLSO).
A Chance fracture involves a horizontal splitting of the vertebra extending from posterior to anterior through the spinous process, pedicles, and vertebral body. Despite the extent of vertebral damage, these fractures tend to be stable. They most commonly occur at the thoracolumbar spine (T12, L1, or L2 level). Chance fractures usually result from an acute hyperflexion of the back and were known as “seat-belt” fractures with the advent of lap seat belts in cars. A head-on collision would cause the passenger wearing a lap-belt to suddenly be flexed at the waist, creating stress on the posterior elements of the vertebra. With the combination of shoulder and lap belts, most Chance fractures seen today result from falls or crush-type injuries where the thorax is acutely hyperflexed.
In the lumbar spine, L1 burst fractures are most common. These frequently result from a fall from a height and will result in an injury partially affecting the conus medullaris and/or the cauda equina (CE). Anterior surgery in the lower lumbar spine is difficult due to the presence of the iliopsoas muscles and the great vessels, and as such, below the level of L3 is rarely performed.
The role and timing of spinal surgery including decompression has not been fully clarified in patients with SCI. Early spinal decompression within 24 hours, and perhaps within 8 hours, may improve neurological recovery, particularly in patients with incomplete injuries (
24,
43). Most previous studies have failed to demonstrate significant neurologic recovery with early surgery (<72 hours) among patients with complete or incomplete lesions (
44,
45). Preliminary results of the STASCIS (Surgical Treatment of Acute SCI Study) trial showed that 25% of patients who received decompressive surgery within 24 hours of their injury experienced a 2-grade or greater improvement on the ASIA scale, compared with 4% of those in the delayed-treatment (>24 hours) group (
46). Further study is needed regarding the timing of surgery and neurological recovery. Data have shown that early surgery reduces LOS in the acutehospital, facilitates rehabilitation, decreases hospital costs, and reduces postoperative complications (
47,
48,
49). The current indication for emergent surgical treatment is progressive neurologic deterioration.
ANATOMY, NEUROANATOMY, AND VASCULAR SUPPLY
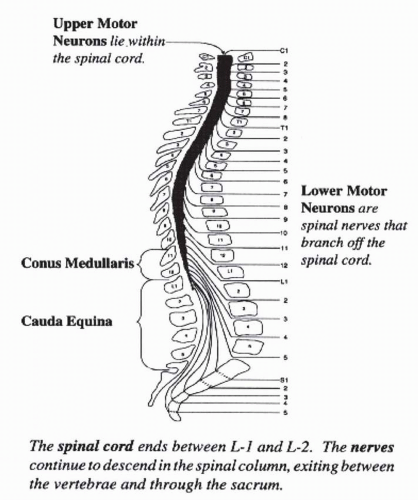
The vertebral column is composed of 7 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 4 coccygeal vertebrae. The spinal cord is protected within the vertebral foramen, and initially occupies the entire length of the vertebral canal. By adulthood, the spinal cord occupies only the upper two thirds of the vertebral column with its caudal end located at the lower border of the first lumbar (L1) vertebra (level of L1-2 intervertebral disc) (
Fig. 27-2). The spinal cord segments, especially in the thoracic and lumbar regions, do not line up with their corresponding vertebral level, and explains why a fracture of T12 for instance results in a L1-2 NLI. At the caudal end, the spinal cord is conical in shape and is known as the conus medullaris. The lumbar and sacral nerve roots descend some distance within the vertebral canal in order to exit from their respective intervertebral foramina. These nerve roots resemble a horse’s tail, and are termed the cauda equina (CE). The lumbar cistern extends from the caudal end of the spinal cord (L2 vertebra) to the second sacral vertebra. The subarachnoid space is widest at this site and is therefore most suitable for the withdrawal of cerebrospinal
fluid (CSF) by lumbar puncture, usually performed between the L3 and L4 lumbar vertebrae.
Thirty-one pairs of spinal nerves (8 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 1 coccygeal pair) emerge from the spinal cord. At and below the thoracic level, the nerve roots exit just caudal to the corresponding vertebra while in the cervical region the nerve roots exit through the intervertebral foramina just rostral to the corresponding vertebra. This is due to the fact that there are eight cervical nerve roots and only seven cervical vertebrae; the C8 nerve root exits through the intervertebral foramen just rostral to the first thoracic vertebra.
The spinal cord has two enlargements: cervical and lumbar. The cervical enlargement includes the C5-T1 nerve roots to form the brachial plexus which innervates the upper extremities (UEs). The lumbar plexus (roots L1 to L4) and lumbosacral plexus (L4 to S2) emerge from the lumbar enlargement and innervate the lower extremities (LEs). The sacral spinal nerves emerge from the conus medullaris and contain PS and somatic motor fibers innervating the muscles of the bladder wall and external sphincter, respectively.
The spinal cord receives its blood supply from one anterior and two posterior spinal arteries (PSAs) as well as anterior and posterior radicular arteries. The anterior spinal artery (ASA) arises in the upper cervical region and is formed by the union of two branches of the vertebral arteries. The ASA supplies the anterior two thirds of the spinal cord including the gray matter and anterior and anterolateral white matter. The ASA varies in diameter according to its proximity to a major radicular artery. It usually is narrowest in the T4-8 region of the spinal cord. There are two PSAs that originate from the vertebral artery. The PSAs supply the posterior one third of the spinal cord consisting of posterolateral and posterior white matter of the spinal cord.
The blood supply from the anterior and posterior arteries is sufficient for the upper cervical segments. Segmental arteries that arise from the aorta supply the ASA and PSAs in the thoracic and lumbar regions. The radicular arteries arise from the vertebral, cervical, intercostal, lumbar, and sacral arteries and supply the remaining segments of the spinal cord. The major radicular artery that supplies the lumbosacral enlargement of the spinal cord is known as the artery of Adamkiewicz. It usually arises from the left intercostal or lumbar artery at the level of T6-L3 and provides the main blood supply to the lower two thirds of the spinal cord. There are less radicular arteries that supply the midthoracic region of the spinal cord and are smaller in diameter and therefore create a “watershed zone” of the spinal cord at this level. With clinical situations where there is low blood flow to the spinal cord (i.e., clamping of the aorta for surgery above the renal artery), this level of the cord is most affected (T4-6 level).
The internal structure of the spinal cord is such that a transverse section of the spinal cord reveals a butterfly-shaped central gray matter surrounded by white matter. The gray matter of the spinal cord contains cell bodies and primarily neurons, dendrites, and myelinated and unmyelinated axons. Autonomic neurons are located laterally and exit by the ventral root and innervate smooth muscle. Lower motor neurons (LMN) are located ventrally, exit by the ventral roots and innervate striated muscle. The white matter consists of ascending and descending bundles of myelinated and unmyelinated axons (tracts or fasciculi). The ascending pathways relay sensory information to the brain while the descending pathways relay motor information from the brain.
Sensory tracts or ascending pathways are composed of axons from peripheral sensory nerves whose cell bodies are located in the dorsal root ganglion (DRG) and ascend toward the brainstem. Receptors for pain and temperature enter the spinal cord and synapse in the dorsal horn of the gray matter. The fibers cross over within one to two vertebral segments, then travel in the lateral spinothalamic tract and ascend to the ventral posterolateral (VPL) nucleus of the thalamus. The fibers then ascend in the internal capsule to reach the postcentral gyrus, which is the primary somatic sensory area of the brain. Pressure and light touch (LT) fibers enter the cord in the same fashion, and pass into the ipsilateral dorsal white column and bifurcate. One branch immediately enters the dorsal horn gray matter, synapses, and crosses over within one to two segments, while the other branch remains ipsilateral, and ascends in the dorsal column for as many as ten spinal segments. The ipsilateral branch ultimately enters the dorsal horn, synapses, and crosses over to join the other branch in the ventral white column, forming the ventral spinothalamic tract. These axons travel in the same pathway as the lateral tract to reach the postcentral gyrus, which interprets these sensations.
The posterior columns transmit three different sensations; proprioception, fine touch, and vibration sense. Their nerve fibers reach the DRG and immediately pass into the ipsilateral dorsal white columns and ascend to the medulla. Axons that enter the cord at the sacral, lumbar, and lower thoracic levels are situated in the medial part of the dorsal column (i.e., the lower part of the body) called the fasciculus gracilis. Those axons that enter at the thoracic (above T6) and cervical levels are situated in the lateral part of the column (from the upper part of the body) and are termed the fasciculus cuneatus. Axons of each fasciculus synapse in the medulla and form a bundle termed the medial lemniscus, which ascends to the postcentral gyrus.
The cerebellum is the control center for the coordination of voluntary muscle activity, equilibrium, and muscle tone. The spinocerebellar tract is a set of axonal fibers originating in the spinal cord and terminating in the ipsilateral cerebellum that conveys information to the cerebellum about limb and joint positions (proprioception).
The lateral corticospinal tract is the main tract for voluntary muscle activity. Its origin is the precentral gyrus of the frontal lobe of the brain. Their axons descend through the internal capsule to the medulla oblongata. Approximately 80% to 90% of the axons cross at the pyramidal decussation to the contralateral side of the medulla and descend in the lateral white columns of the spinal cord, in the lateral corticospinal tract. At each level of the spinal cord, the axons from the lateral tract peel off and enter the gray matter of the
ventral horn and synapse with secondary neurons. The 10% to 20% of uncrossed axons that continue down on the same side of the cord travel in the ventral corticospinal tract. The axons of the ventral tract then cross over at the corresponding level of muscles that it innervates. Both tracts travel from the precentral gyrus to the ventral horn as a single uninterrupted neuron and are termed upper motor neurons (UMN), while the secondary neurons that they synapse on, are termed lower motor neurons.
NEUROLOGICAL ASSESSMENT
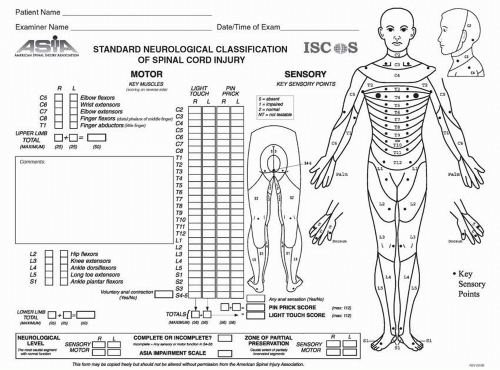
The most accurate way to document impairment in a person with a SCI is by performing a standardized neurological examination, as endorsed by the International Standards for Neurological Classification of SCI Patients (
50). These standards provide basic definitions of the most common terms used by clinicians in the assessment of SCI and describe the neurological examination. Key terms are defined in
Table 27-2. The examination is composed of sensory and motor components, and is performed with the patient in the supine position to be able to compare initial and follow-up exams. The information from this examination is recorded on a standardized flow sheet (
Fig. 27-3) and helps determine the sensory, motor, and NLI, sensory and motor index scores, and to classify the impairment. An online course for examination and classification utilizing the Standards is available through the ASIA website (www.asia-spinalinjury.org).
Sensory exam: The sensory exam is performed separately for LT and pin prick (PP) modalities. Each of 28 dermatomes (
Fig. 27-1) is tested and graded 0 for absent, 1 for impaired, 2 for normal (or intact), or NT for not testable. The face is used as the reference point for testing sensation in each dermatome. A grade of 2 indicates the sensation is equal to that of the face. For the pin exam, a grade of 1 indicates the ability to distinguish sharp from dull; however, the sensation is qualitatively different as compared to the face (i.e., either less sharp or hyperesthetic). If the patient cannot distinguish the sharp from the dull aspect of the safety pin used for testing, then the score is 0. In questionable cases, eight out of ten correct answers are suggested as a standard for accuracy. A score of 0 is also given if there is no sensation. For the LT exam, a cotton tip applicator is used. A score of 1 is recorded if the sensation is less than on the face and a 0 if there is no sensation at all. The lowest sacral segment, S4-5 (anal musculocutaneous junction), should be tested with the pin and cotton swab as well. It is important to document the modality of sensation spared, as preservation
of pin sensation in the lower sacral segments yields a better prognosis for neurological recovery.
To test for deep anal sensation (DAS), a rectal digital exam is performed. The patient is asked to report any sensory awareness, touch, or pressure, with firm pressure of the examiners’ digit on the rectal wall. DAS is recorded as either present (yes) or absent (no).
The maximum sensory score is 112 (calculated by adding the scores from the 28 dermatomes—maximum score of 56 for each side of the body) for LT and pin sensation. The sensory level is defined as the most caudal level where sensation for LT and PP are both graded as 2 (normal) for both sides of the body. If the LT level is C6 and PP is C5, the overall sensory level is C5. In a case where sensory loss begins at, or just above the nipple line (T4 dermatome), often a patient is credited with the T3 dermatome being spared. If the sensation is absent in the T1 and T2 dermatomes despite the presence of some sensation at the T3 dermatome it is recommended that the T3 dermatome be scored as absent. It is felt that this sparing above the nipple line is from C4 innervation.
Motor exam: The motor exam is conducted using conventional manual muscle testing (MMT) technique (on a scale from 0 to 5) in ten key muscle groups, five in the upper limb (C5-T1 myotomes) and five in the lower limb (L2-S1), on each side of the body (
Table 27-2). Key muscles were chosen based upon their myotomal innervations and ability to be tested in the supine position. Most muscles are innervated by two root levels (e.g., the elbow flexors are innervated from C5 to C6). When a key muscle tests initially as a grade 5, it is presumed to be fully innervated by the contributions from the two roots. If a muscle initially grades a three fifth, it is presumed to have full innervation of its more proximal segment (in the case of the elbow flexors, innervation from the C5 myotome). The maximum motor index score is 100 (calculated by adding the scores—maximum of 50 for each side of the body). Voluntary anal contraction is tested by sensing contraction of the external anal sphincter around the examiner’s finger and graded as either present or absent.
When examining a patient with an acute injury below T8, the hip should not be flexed passively or actively beyond
90 degrees as this may place too great a kyphotic stress on the lumbar spine. Therefore, it may only be possible to test hip flexor muscle strength isometrically. While asking the patient to lift the leg straight off the bed, the patient’s movement is resisted and the examiner’s judgment is required to grade the muscle force as 2 through 5.
If a muscle’s range is limited by contracture that exceeds 50% of the normal ROM, the muscle is to be listed as NT (not testable); if less than 50% loss of range—the MMT scoring can be applied. NT is also used if the muscle cannot be tested; that is, a cast in place limiting MMT. If spasticity interferes with the exam significantly, then NT should be documented. If pain limits full patient effort and the examiner feels that the initial contraction given represents normal strength, the muscle should be graded as 5*, while indicating the reason for this scoring (e.g., pain).
The motor level is defined as the most caudal motor level with a score of ≥3, with the more cephalad key muscles grading a 5. For injuries with no corresponding motor level (i.e., above C4, T2-L1), the last normal sensory level is used. For example, a person with normal strength in all key muscles of the UEs, 0/5 strength in the key muscles of the LEs, with a T4 sensory level, would be assigned a motor level of T4. Similarly, in a case where the elbow flexors (C5 level) grade 3/5 on both sides, with a sensory level on the left at C4 and on the right C3; the motor level on the left would be C5 and on the right C3. This is due to the C4 dermatome on the right being scored as impaired—it is presumed that the C4 myotome is also impaired. Therefore, the motor level is designated as C3, since the patient does not meet the criteria of having a key muscle ≥3/5, with the levels above (in this case C4) scoring as normal. On the left side, the C4 dermatome is normal so the C4 myotome is considered normal and as a result, the left motor level is C5.
The NLI is the most caudal level, at which both motor and sensory modalities are intact on both sides of the body. The motor and sensory levels are the same in less than 50% of complete injuries, and the motor level may be multiple levels below the sensory level at 1-year postinjury (
51). In cases where there is no key muscle level available (i.e., cervical levels at and above C4; T2-L1; and sacral levels below S2), the NLI is that which corresponds to the sensory level.
If there are non-SCI-related causes of weakness, this should be documented and taken into account when classifying the injury. For example, in a patient with a T8 fracture and complete paraplegia who also has a left brachial plexus injury, notation should be made that the sensory and motor deficits in the left arm are due to brachial plexus injury, not SCI, and the patient may still be classified with a NLI of T8. The motor level and UE motor index score better reflect the degree of function as well as the severity of impairment and disability, relative to the NLI, after motor complete tetraplegia (
51).
AIS Classification
The International Standards have evolved over the past 25 years and have become accepted as the most appropriate method to describe the neurological impairment of SCI for clinical and research use and have been incorporated into the International Core SCI Data Set. In 1982, the ASIA first published the
Standards for Neurological Classification of SCI, adopting the Frankel Scale (
52). The Standards were replaced in 1992 by the AIS (
53) and revised a number of times since (1996 and 2000) with reprinting in 2002 and 2006 (
50,
53,
54).
The patient’s injury is classified utilizing the AIS, separating the injury into a neurologically complete versus incomplete injury. A neurologically complete injury is defined as an injury with the individual having no “sacral sparing.” Sacral sparing refers to having one or more of the following residual findings: LT or PP in the S4-5 dermatome (can be on either side, impaired or intact); DAS or voluntary anal contraction preserved. If any of these components are present, the individual has sacral sparing and therefore has a neurologically incomplete injury. Patients who have an incomplete injury initially (i.e., sacral sparing) have a significantly better prognosis for motor recovery than those without preservation of the lower sacral segments.
Table 27-3 describes the steps to classify the SCI and
Table 27-4 outlines the AIS. A reference manual and training video are available that provide a detailed explanation of the examination elements (ASIA, Atlanta, GA. www.asia-spinalinjury.org). The
zone of partial preservation (ZPP) refers to the dermatomes and myotomes caudal to the NLI that remain partially innervated in persons with a neurologically complete injury (AIS A). The ZPP should be recorded as the most caudal segment with some sensory and/or motor sparing but only in persons with a neurologically complete injury.
A neurologically complete injury is classified as AIS A. Persons with sensory sacral sparing are classified as an AIS B. To be classified with a motor incomplete injury (AIS C or D), the subject must have either (a) voluntary anal sphincter contraction or (b) sensory sacral sparing with sparing of motor function more than three levels below the
motor level (
50,
54). To differentiate an AIS C from D, the individual with a motor incomplete injury AIS D has at least half of key muscles below the
NLI with a muscle grade of 3 or more (AIS C would be less than half). It is important to recognize the distinction using the motor level to determine if one with sensory sacral sparing has a motor incomplete injury (AIS B vs. C), yet uses the NLI when differentiating an AIS C from D.
Incomplete SCI Syndromes
Incomplete SCI syndromes include central cord (CCS), Brown-Sequard, anterior cord, conus medullaris, and CE syndromes (CES), and can result from traumatic as well as NT injuries (
55). CCS is the most common, accounting for approximately 50% of incomplete injuries and 9% of all traumatic SCI. CCS is characterized by motor weakness in the UE greater than the LE, in association with sacral sparing. CCS most frequently occurs in older persons with cervical spondylosis who suffer a hyperextension injury from a fall, but it may also occur in persons of any age and is associated with other etiologies, predisposing factors, and injury mechanisms (
56). The postulated mechanism of the injury involves compression of the
cord both anteriorly and posteriorly by degenerative changes of the bony structures, with inward bulging of the ligamentum flavum during hyperextension in an already narrowed spinal canal (
55,
56).
It has been postulated that the pathophysiology of the upper limbs being more affected than that of the lower limbs is that the motor tracts of the upper limbs are located more centrally, while those of the lower limbs are located more peripherally in the spinal cord (
56). MRI evidence has revealed that this clinical pattern may not be based on locations of the arm and leg fibers within the corticospinal tract, but rather that this tract carries fibers that mainly innervate distal limb musculature, and therefore, the functional deficits are more pronounced in the hands when the tract is the primary site of damage (
57).
CCS generally has a favorable prognosis for functional recovery. Recovery occurs earliest and to the greatest extent in the LEs, followed by bowel and bladder function, proximal UE, and then distal hand function. Prognosis for functional recovery of ambulation, ADL, and bowel and bladder function is dependent upon the patient’s age (< or >50 years of age), with a less optimistic prognosis in older patients (>50 years old) relative to younger patients (
55,
58,
59,
60). Specifically, younger patients are more successful in becoming independent in ambulation (87% to 97% vs. 31% to 41%), bladder function (83% vs. 29%), and dressing (77% vs. 12%) (
58) than older patients. Older newly injured individuals, however, with an initial classification of AIS D tetraplegia, have a good prognosis for recovery of independent ambulation (
60).
Brown-Sequard syndrome (BSS) is defined as a lesion similar to a hemisection of the spinal cord, and accounts for 2% to 4% of all traumatic SCI (
55,
61,
62,
63). In the classic presentation, there is (a) ipsilateral loss of all sensory modalities at the level of the lesion; (b) ipsilateral flaccid paralysis at the level of the lesion; (c) ipsilateral loss of position, sense, and vibration below the lesion; (d) contralateral loss of pain and temperature below the lesion; and (e) ipsilateral motor loss below the level of the lesion. This is due to the crossing of the spinothalamic tracts in the spinal cord, as opposed to the corticospinal and dorsal columns that cross the brain stem. The pure form of BSS is rare and the Brown-Sequard Plus Syndrome is more common (BSPS) (
64), which refers to a relative ipsilateral hemiplegia with a relative contralateral hemianalgesia. Although BSS has traditionally been associated with knife injuries, a variety of etiologies, including closed spinal injuries with or without vertebral fractures may be the cause (
64,
65).
Recovery usually takes place in the ipsilateral proximal extensors and then in the distal flexors (
66,
67). Motor recovery of any extremity having a pain/temp sensory deficit occurs before the opposite extremity. Overall, patients with BSS have the greatest prognosis for functional outcome of the incomplete syndromes, as 75% to 90% of patients ambulate independently at discharge from rehabilitation and nearly 70% perform functional skills and ADL independently (
62,
64). In predicting outcome, when the upper limb is the predominant site of weakness, patients are more likely to ambulate at discharge. Recovery of bowel and bladder function is also favorable.
The
anterior cord syndrome involves a lesion affecting the anterior two thirds of the spinal cord while preserving the posterior columns. This may occur with flexion injuries, from retropulsed disc or bone fragments compressing the cord, direct injury to the anterior spinal cord, or with lesions of the ASA which provides blood supply to the anterior spinal cord (
55,
62,
68). There is a variable loss of motor as well as PP sensation with a relative preservation of LT, proprioception, and deep-pressure sensation. Patients usually have a 10% to 20% chance of muscle recovery (
69).
Posterior cord syndrome (PCS) has been omitted from recent versions of the International Standards, and is the least common of the SCI clinical syndromes with an incidence of less than 1% (
55,
62). There is a loss of proprioception and vibration sense, but with preservation of muscle strength, temperature, and pain sensation due to a selective lesion of the posterior columns. PCS has been linked to neck hyperextension injuries, PSA occlusion, tumors, disk compression, and vitamin B
12 deficiency. Prognosis for ambulation is poor, secondary to the proprioceptive deficits.
Conus medullaris and Cauda Equina (CE) injuries: The conus medullaris, which is the terminal segment of the adult spinal cord, lies at the inferior aspect of the L1 vertebrae. The segment above the conus medullaris is termed the epiconus, consisting of spinal cord segments L4-S1. Lesions of the epiconus will affect the lower lumbar roots supplying muscles of the lower part of the leg and foot, with sparing of reflex function of sacral segments. The bulbocavernosus (BC) reflex and micturition reflexes are preserved, representing an UMN or suprasacral lesion. Spasticity will most likely develop in sacral innervated segments (e.g., toe flexors, ankle plantar flexors, and hamstring muscles). Recovery is similar to other UMN spinal cord injuries.
Conus medullaris lesions present with UMN and LMN aspects. Lesions affecting neural segments S2 and below will present with LMN deficits of the anal sphincter and bladder due to damage of the anterior horn cells of S2-4. Bladder and rectal reflexes are diminished or absent, depending on the exact level and extent of the lesion. Motor strength in the legs and feet may remain intact if the nerve roots (L3-S2) are not affected, that is, “root escape.” Trauma and tumors are among the most common etiologies responsible for this condition.
Injuries below the L1 vertebral level usually affect the CE or nerve rootlets supplying the lumbar and sacral segments producing motor weakness and atrophy of the LEs (L2-S2) with bowel and bladder involvement (S2-4), impotence, and areflexia of the ankle and plantar reflexes. CES is a LMN injury. In CES there is a loss of anal and BC reflexes. CE injuries have a better prognosis relative to UMN injuries for neurological recovery, most likely due to the fact that the nerve roots are more resilient to injury as they are histologically peripheral nerves and therefore regeneration can occur. CE injuries may represent a neuropraxia or axonotmesis, and demonstrate progressive recovery over a course of weeks and months. CES can occur as a result of trauma, tumors, spinal stenosis, disc compression, infection, or postsurgical epidural hematoma (
55).
Separation of CE and conus lesions in clinical practice is difficult, because some of the clinical features of these lesions overlap. Pain is uncommon in conus lesions but is frequently a complaint in CES. Sensory abnormalities occur in a saddle distribution in conus lesions and, if there is sparing, there is usually dissociated loss with a greater loss of pain and temperature while sparing touch sensation. In CE lesions, sensory loss occurs more in a root distribution and is not dissociated.
FUNCTIONAL EVALUATION
The International Standards (
50) are the most widely accepted instrument of impairment, and interrater reliability is very good overall (
70). The Modified Benzel Scale is a 7-grade scale used in the Sygen studies (
71) that expands the AIS D category into three separate grades. The International Classification for Surgery of the Hand in Tetraplegia (
72) is most commonly used when dealing with upper limb reconstruction procedures. A committee has developed a classification for autonomic functions, including blood pressure (BP), heart rate, and temperature regulation; bladder function; bowel function; and sexual function (
70,
73).
Functional limitation in SCI can be measured by the Grasp and Release Test that evaluates the impact of implanted upper limb prosthesis (
74) or the capabilities of UE instrument
(
75,
76). To measure walking in a standardized environment as it relates to walking, the Timed Get Up and Go test, 6-minute walk test or 10 m walk have been utilized (
70,
77). The Walking Scale for SCI (WISCI) is a valid scale that ranks walking based on various combinations of braces, assistive devices, and level of personal assistance (
78,
79).
Activity performance, what an individual can do in his/her environment, can be measured by the functional independence measure (FIM), the Canadian Occupational Performance Measure, the Quadriplegic Index of Function (QIF), and the spinal cord independence measure (SCIM) (
70,
80,
81,
82,
83,
84). The FIM as a generic instrument has shortcomings when applied to SCI and while the FIM was added to the Standards in 1992 (
53), it was removed in the 2000 revisions (
54). The QIF and SCIM are more sensitive and a better indicator of motor recovery as compared to the FIM, since it can reflect small gains in function which parallel small strength gains (
84,
85). The QIF is limited in use to persons with tetraplegia and has been used sparingly. The SCIM addresses indoor and outdoor activities (
86) and is gaining in popularity.
Individuals with the same SCI level and severity may have different levels of activity performance due to differences in adaptive equipment, personal assistance, and accessibility of their environment. Impairments are not highly correlated with community integration, that is, the inability to walk does not prevent one from working. The CHART is the most commonly used measure of community functioning (participation) for persons with SCI (
87). QOL is not related to impairment, but rather is highly associated with social support, community integration, and resumption of life and family roles (
88).
PROGNOSTICATING NEUROLOGICAL RECOVERY
Prognosticating neurological recovery early after a traumatic SCI is important to provide accurate information to patients and their families and to guide the rehabilitation course. The major factors in predicting recovery early after a traumatic SCI include the initial NLI, the initial motor strength, and most importantly, whether by examination the injury is classified as neurologically complete or incomplete (
89). This information is best provided by performing the neurological physical examination using the International Standards (
90). The examination at 72 hours postinjury is superior to that at 24 hours postinjury for predicting recovery (
91,
92).
Between 30% and 80% of patients with initially complete tetraplegia will recover one root level of function. Most UE recovery occurs during the first 6 to 9 months, with the greatest rate of change during the first 3 months. Motor recovery can continue during the second year, especially for patients with initially 0/5 strength. The initial strength of the muscle, immediately below the motor level, is the most significant predictor of achieving antigravity strength by 1-year postinjury (
93,
94,
95). If the first level below the NLI has 0/5 strength at 72 hours to 1 week, approximately one third of patients will recover to 3/5 strength in that muscle. If there is some initial strength present, approximately 75% to 95% of patients will regain antigravity strength at 1 year. At 1 month, if there is no motor strength in the first level below the NLI, then only 27% will improve to 3/5 by 1 year (
94). Presence of sensation in the dermatome corresponding with that motor level, improves the chances of recovery. The faster an initial 0/5 muscle starts to recover some strength, the better the prognosis for recovery. Only a small percentage of subjects have motor recovery below the first level caudal to the motor level. Recovery at the C4 level to the C5 level, both motor and sensory, may be less and slower than at the lower cervical segments, especially if there is initially no strength at the C5 myotome (
96).
Persons with incomplete tetraplegia have a better prognosis for recovery (
97,
98). UE motor recovery is approximately twice as great in incomplete tetraplegia as in complete tetraplegia, with the potential for varying degrees of LE motor recovery and functional ambulation. For patients who are sensory incomplete initially, the prognosis for motor recovery is more favorable in those with sparing of pin sensation rather than LT sensation alone (
92,
97,
98,
99). The basis of a more favorable outcome for pin sparing may be explained by the close anatomical relationship of the motor tracts (lateral corticospinal tract) to the sensory tracts carrying pain and temperature fibers (lateral spinothalamic tract). Ambulation can be predicted by having PP sensation sparing in 50% or more of the dermatomes in the LE (L2-S1) (
99). Up to 40% of patients initially classified as AIS B will improve to AIS C and up to 40% to AIS D (
92). Functional and neurological recovery is even more favorable for patients with an initial motor incomplete injury. Approximately 60% to 80% of patients initially classified as AIS C will improve to AIS D. The majority of motor recovery occurs within the first 6 months after injury, and the early return of motor function suggests a better functional outcome. Motor recovery in the UEs and LEs occurs concurrently, rather than sequentially.
In complete paraplegia, the potential for LE motor recovery improves with lower initial NLI; 15% of patients with a NLI between T9 and T11, and 55% of those with an initial NLI below T12, demonstrate recovery of some strength in the lower limbs (
100). Most movement gained is in the proximal LE musculature and may represent recovery of partially injured lumbar roots or “root escape.”
Individuals with incomplete paraplegia have the best prognosis for LE motor recovery and ambulation (
101). Eighty percent of individuals with incomplete paraplegia regain antigravity hip flexors and knee extensors at 1 year. Individuals with no LE motor control at 1 month may still show significant return by 1 year.
AIS A Conversion and Late Recovery
The vast majority of patients initially designated as having a complete injury will remain an AIS A. There are older reports of up to 10% of initial AIS A converting to AIS B and 10% regaining some motor function at later follow-up (
92,
102). Burns et al. reported that at year 1 or later, less than 7% (2/30)
of AIS A subjects initially tested within 2 days without factors affecting exam reliability, converted to AIS B status, and none developed volitional motor function below the zone of injury (
103). Aspects of an early examination that may render it unreliable include factors that may affect cognition (i.e., TBI, drugs) and communication barriers (i.e., on ventilator). More recent literature has reported greater amount of conversion from initial to follow up examinations. A comprehensive review of the existing literature by the International Campaign for Cures of Spinal Cord Injury Paralysis (ICCP) reported a conversion rate of ˜20% at one year post-injury, with 10% converting to AIS B and an additional 10% regained some motor function (AIS C and D) (
104). The conversion rate of AIS A was twice as high for persons with tetraplegia compared to paraplegia. A recent evaluation of the European Multi-center study on human spinal cord injury (EM-SCI) reported that 70% of persons with initial (within 15 days) AIS A remained AIS A at one year, with 17.3% improving to AIS B, 5.8% to AIS C, and 7.2% to AIS D (
105). Late conversion (i.e., change in status from a neurologically complete to incomplete injury after 30 days) has been reported to occur in 4% to 10% of cases (
92,
94,
106,
107). Late conversion can occur even years after injury, although usually only to AIS B with very few (<2%) to motor incomplete status, or C (
108).
Other Predictors of Neurological Recovery
The presence of spinal shock may play a role in prognosis; for the same degree of SCI, the presence of spinal shock implies a more rapid evolution of injury and a poorer prognosis. The order that reflexes return in the postinjury period may help prognosticate outcome (
24,
109). Absence of the BC reflex (S3-4 roots) or the anal reflex (S2-4 roots) after the acute period (24 to 72 hours) suggests injury to the conus medullaris or CE (i.e., LMN injury). As such, prognosis regarding recovery and also the potential use of rehabilitation intervention (e.g., electrical stimulation [ES]) can be determined. The delayed plantar response, which may be the first of all reflexes to return, occurs within hours or days following SCI, and its persistence shows a high correlation with complete injuries and a poor prognosis for LE motor recovery and function (ambulation) (
109,
110). The presence of the crossed adductor response to patellar tendon taps in the acute stage is highly predictive of functional motor recovery (
111). Older individuals may have a less favorable outcome in regard to neurological recovery, functional ambulation, and bowel and bladder independence than younger patients with similar severity of the injury, and they have more associated medical complications (
112).
Radiological and electrodiagnostic results early after injury assist in confirming the prognosis obtained from the clinical evaluation. The type of fracture often correlates with the severity of the injury. Bilateral cervical facet dislocation, thoracolumbar flexion/rotation injuries, and transcanal bullet locations more commonly occur with neurologically complete injuries. An intramedullary hemorrhage correlates with a more severe initial neurologic deficit, and carries a poorer prognosis. Hemorrhage location corresponds anatomically to the level of the neurologic injury. The greater the extent of cord signal abnormality on MRI, the greater the chance of having a complete injury (
113,
114,
115,
116). Lesions in patients with incomplete SCI (AIS B through E) were found to have a mean length of ≤20 mm, whereas those in patients with neurologically complete injuries had a mean length of 40 mm (
116). The presence of cord edema greater than 1 vertebral body segment is also a poor prognostic finding. Smaller degrees of cord edema and especially the absence of abnormal signal intensity on MRI in the spinal cord are considered positive predictors for neurologic recovery. In the chronic stage after SCI, persons with persistent cord signal changes on MRI demonstrate little improvement in AIS grades relative to the improvements of patients with resolution of signal abnormalities.
Electrophysiological techniques include nerve conduction studies, late responses (H-reflex and F-wave), somatosensoryevoked potentials (SSEP), motor-evoked potentials (MEP), and sympathetic skin responses (SSR) that can supplement clinical and neuroradiological findings (
89,
117,
118,
119,
120,
121,
122). These tests, however, are most useful in differentiating lesions between the central and peripheral nervous system. They may also help in diagnosing a neurologically complete versus incomplete injury in uncooperative or unconscious patients, or to rule out a conversion disorder, since they do not require the cooperation of the patient. They are not a routine part of the acute investigation of a newly injured individual to offer prognosis for neurological or functional outcome.
Nontraumatic Spinal Cord Injury
NT SCI is a growing population for admission to inpatient rehabilitation and includes such etiologies as spinal stenosis with myelopathy, spinal cord compression from a neoplasm, multiple sclerosis (MS), transverse myelitis (TM), infection (viral, bacterial, fungal, parasitic), vascular ischemia, radiation myelopathy, motor neuron diseases, syringomyelia, vitamin B12 deficiency, and others. Spinal stenosis and spinal cord tumors are the most common causes of NT SCI presenting for inpatient rehabilitation in the United States (
123,
124).
Details regarding most of these disorders are covered elsewhere in the text. TM is an inflammatory disorder of the spinal cord, with a female to male ratio of 4:1 that peaks in the second and fourth decades. TM most commonly affects the thoracic spine (50%) and diagnosis is best established by MRI. The time course of progression of symptoms that include back pain, motor weakness (the UEs are less often involved), abnormal sensations, and neurogenic bowel and bladder, is from hours to weeks. Although some patients recover with minor or no residual problems, most remain with residual permanent impairments. Poor recovery is predicted by rapid progression, back pain, and spinal shock (
125). Most patients will have only one episode of TM, but a small percentage may have a recurrence.
SCI from epidural abscess is most commonly seen in immunocompromised and diabetic patients. Staphylococcus aureus is the most common pathogen. Epidural hematoma can be associated with anticoagulation, vascular malformations, or myelodysplastic diseases. Arterial disease from a thrombus or embolism to the spinal arteries or from other vascular diseases, including vasculitis and diabetes, or from thoracoabdominal aneurysm repair (reported in 9% to 18% of cases), may cause spinal cord ischemia/infarction.
Late radiation myelopathy is a delayed complication of radiation to lesions of the spine or adjacent tissues that develops months or years after treatment. The incidence of this complication is correlated with the total radiation dose, the dose fraction, and the length of the spinal cord irradiated. Clinically, there is weakness, loss of sensation, and sometimes a Brown-Séquard like syndrome develops. The injury predominantly affects the white matter in the lateral spinal cord. The diagnosis of late radiation myelopathy remains one of exclusion and prognosis for significant recovery is poor.
Spinal cord tumors can be primary or metastatic, intradural, or extradural (see
Chapter 43 for details). The majority of spine and spinal cord tumors are metastatic in origin and 95% are extradural. Approximately 70% of spinal metastasis occurs in the thoracic spine, with clinical presentation of pain, which is typically worse at night and in the supine position. Neurological changes including weakness, sensory loss, and bowel/bladder involvement indicates cord involvement. The most common sources of secondary tumors are metastatic lung, breast, and prostate. The strongest predictors of functional outcome are the tumor type and the preoperative neurological status (
126).
As compared to persons with traumatic-related SCI, individuals with NT SCI are more likely to be older, female, married, and retired. NT SCI is more common than trauma in persons over 40. Persons with NT SCI usually have a less severe neurological impairment as compared with traumatic SCI, as they more often present with motor incomplete (90%) lesions (
127). There is a lower incidence of secondary medical complications including spasticity, orthostasis, deep vein thrombosis (DVT), PUs, autonomic dysreflexia (AD), and wound infections during rehabilitation in patients with NT SCI (
128,
129,
130). However, because cervical stenosis and neoplastic SCC has a peak incidence between the ages of 50 and 70 years, these individuals may have other premorbid medical issues that may impact their rehabilitation. No difference is noted for depression, urinary tract infections (UTIs), heterotopic ossification (HO), pain, or gastrointestinal (GI) bleeds.
No statistical differences were found in acute care LOS and admission to rehabilitation FIM scores (
123,
127,
131) in patients with NT SCI relative to traumatic SCI. Inpatient rehabilitation LOS are shorter for persons with NT injury secondary to tumors, but FIM efficiency and home discharge rates are overall comparable to traumatic SCI. There is a favorable discharge to home for patients who have an incomplete injury, are married, have an established bowel and bladder management program, intact skin, male gender, and who are cognitively intact.
Rehabilitation of SCI
Rehabilitation begins in the intensive care setting and includes addressing the SCI-specific needs to help the individual meet their potential in terms of medical, physical, social, emotional, recreational, vocational, and functional recovery (
132). If early medical complications can be prevented, the inpatient rehabilitation course is facilitated, and the total cost of care is lessened.
The medical aspects of the SCI specialist’s acute recommendations can be formulated into a problem list (
Table 27-5). The most important aspects include bowel, bladder and pulmonary management, deep venous thrombosis, GI prophylaxis, and proper positioning in bed to prevent contractures and skin breakdown, with the proper turning frequency (at least every 2 hours). Once the spine is stabilized by surgery or orthoses, therapy and nursing will incorporate ROM that will help prevent contractures. The shoulder, elbow, hip flexors, and heel cords are most important to range because they are the most
frequently observed contractures on presentation to the acute rehabilitation unit and can potentially serve as a source of pain and functional limitation. Resting splints for paralyzed UEs help prevent contractures and increase comfort. Functional splints may also be useful for feeding and other self-care skills in the early period. For persons with high level tetraplegia who have undergone tracheostomy, early introduction of communication aids is important. Once medically stable, the patient should be transferred to a specialized spinal cord rehabilitation unit.
A specialized SCI center is most beneficial to provide for comprehensive management as it offers access to experienced SCI physicians and therapists, including psychology, vocational and SCI educational services, an active peer support program, and an opportunity to undergo rehabilitation with other patients who have similar impairments (
133). A comprehensive SCI education program is essential for educating the patients and their families about SCI-related issues. Additional opportunities available at larger SCI centers include availability of trial equipment for mobility and accessibility to high level assistive technology. The interdisciplinary approach of the rehabilitation team, including the patient and family, is important for the optimal care of the individual with SCI. A sample rehabilitation prescription is listed in
Table 27-6. As the LOS shortens in acute rehabilitation, coordination and communication with the entire team is needed to allow for a timely and safe discharge. Frequent team conferences with an early home evaluation are recommended.
Functional Goals
Once the patient’s motor level of injury, AIS, and prognosis for neurological recovery are determined at the onset of rehabilitation, short- and long-term functional goals are formulated and a therapy prescription established. The CPG on outcomes following traumatic SCI delineates expected (at 1 year postinjury) functional outcomes, and equipment needs based on the level of injury for persons with SCI with a motor complete injury (
134).
Table 27-7 lists the expected functional outcome at 1 year postinjury and
Table 27-8 lists equipment usually prescribed for persons for each level of injury.
The projected long-term goals are a starting point for the rehabilitation prescription. The rehabilitation program should be individualized to meet each person’s strengths, weaknesses, and individual circumstances. Monitoring progress at the team conference, with the patient as an active participant, helps to identify limiting factors along with the patients’ additional needs. Discharge planning should be discussed as early as the first team conference, assuring a timely, but most importantly, a safe discharge. The ideal outcome may not always be achieved for each patient, as there is a significant amount of variability in individual outcomes despite similar levels of injury based upon the age, gender, and medical comorbidities.
C1-4 Level
Persons with motor levels above C3 will usually require long-term ventilator assistance, whereas most individuals with lesions at C4 will be able to wean off the ventilator. Respiratory equipment including a ventilator, a method for secretion management (i.e., suction machine or mechanical insufflator/exsufflator), back-up ventilator batteries, and a generator in case of power failure, should be obtained. Contact should be made with the local power company and emergency services to alert them of the patient’s needs prior to discharge.
Rehabilitation goals for persons with high cervical SCI primarily include prevention of secondary medical complications, education and training of the patient and family members, prescription of appropriate durable medical equipment (DME), and environmental modification. The patient should be independent in instructing others in providing care including weight
shifts, ROM, positioning, donning orthoses, transfers, and in setting up their environmental control unit (ECU). Additional goals include independence in power wheelchair mobility, using breath control, mouth stick, head array, tongue, or chin control mechanisms. The wheelchair should be equipped with a pressure relief cushion and recline and/or tilt features for independent pressure relief. If the patient can control a power chair, then both a power chair and a manual positional wheelchair with a high back and tilt or recline should be prescribed for use as a back-up wheelchair for assisted mobility in the home and in the community as needed (
136). Once properly set up, persons at these levels of injury should be independent in using assistive technology. This includes lower level technology devices (i.e., adapted phones and page turners) and higher level devices (known as electronic aides of daily living [e-ADL]) that control one or more electronic appliances (i.e., television, radio, lights) via voice activation or switch access. These will allow for independence in communication and controlling their local environment. A type of lift to assist in transfers and a padded commode/shower chair should be prescribed. An attendantoperated van with a lift and tie-down, or accessible public transportation is necessary for community mobility.
The patient’s family and social support, financial resources, personal preferences, educational, vocational and avocational goals, and living arrangements after discharge must be fully considered during rehabilitation. Post-acute medical, psychosocial, and rehabilitation care should be prescribed in the home or outpatient setting. Although the evaluation process requires input from many specialist team members, the physiatrist is ultimately responsible for medical justification of all equipment decisions and should be directly involved in the DME evaluation and prescription process.
Restoring respiratory function for persons with high level of injuries (C2 and above) have been achieved by the use of various ES (see later under pulmonary section). These procedures may assist in weaning off of the ventilator, and enhance speech production and improve QOL (
135,
136).
Persons with a NLI at C4 who have some elbow flexion and deltoid strength may be able to use a mobile arm support (MAS) or balanced forearm orthosis (BFO) to assist with feeding, grooming, and hygiene. Once the elbow flexors have antigravity strength with adequate endurance, these devices are
no longer needed. A long straw or a bottle that the person can easily access to drink fluids should be obtained as early as possible.
The benefit of specialized acute inpatient rehabilitation for persons with such high levels of injury is justifiable despite their inability to initially tolerate 3 hours a day of therapy and having what may seem as limited goals. The SCI medical and nursing care during the first few months after injury are crucial for monitoring, treating, and preventing medical complications that can lead to future morbidity and mortality. Patient and family education, emotional and social support, and exposure to advanced technology that may allow independence in the proper environment (i.e., power mobility, assistive technology) may be the difference between returning to their family/community and living in a long-term facility.
C5 Level
The C5 motor level adds the key muscle group of the elbow flexors (biceps), as well as the deltoids, rhomboids, and partial innervation of the brachialis, brachioradialis (BR), supraspinatus, infraspinatus, and serratus anterior. It is important during the acute period after SCI to prevent elbow flexion and forearm supination contractures caused by unopposed flexion activity by stretching, splinting, and if needed antispasticity injections. A long opponens splint, with a pocket for inserting different utensils (i.e., Universal cuff), is important to assist with many tasks including feeding, hygiene, grooming, and writing. Most functional activities will require the use of assistive devices; however, tendon transfers may be considered after neurological recovery is complete.
The addition of the elbow flexors should allow for use of a joystick for a power wheelchair and can allow manual wheelchair propulsion on level surfaces with either rim projections (lugs) or plastic-coated handrims with a protective glove. A power wheelchair, with a power recline and/or tilt mechanism, is usually still required in addition to the manual wheelchair. Push rim-activated power-assist wheelchairs may also be advantageous. These are manual wheelchairs that have a motor linked to each rear hub. With each manual propulsion by the user, there is supplementary power provided by the motor. Therefore, the force required by the user for propulsion over the same distance is decreased when compared to a regular manual wheelchair. This feature is particularly useful for those with tetraplegia or those with paraplegia, and overuse injury causing shoulder pain. The use of power-assist wheelchairs can improve the ADL in persons with tetraplegia, when compared to the use of regular manual wheelchairs (
137).
Another recent significant wheelchair development is the Independence iBOT 4000 Mobility System. One of its unique functions is the ability to negotiate curbs and stairs. The iBOT is not necessarily appropriate for all power wheelchair users, as there are certain prerequisites that the user must meet. In order to climb the stairs, adequate UE function to grab the handrail and to stabilize the iBOT while initiating the climbing movement is necessary or this can be performed with the assistance of a caregiver.
Persons with this level of injury will require almost total assistance for their bowel program. A padded commode/shower chair is recommended as the gravity from the upright position assists with the program and the padding helps to prevent skin breakdown. Bladder management is a decision based upon discussion with the SCI specialist and urologist, urodynamic results, amount of assistance available, and lifestyle circumstances. Intermittent catheterization (IC) usually cannot be performed independently, and will need to be performed by another person. If using a leg drainage bag, electronic devices to help empty the bag are available. Driving a specially modified van is possible at this level, with a lift for access allowing the patient to be fully independent in this activity.
C6 Level
The C6 level adds the key muscle group that performs wrist extension (extensor carpi radialis), as well as partially innervating the supinator, pronator teres, and latissimus dorsi. Active wrist extension can allow for tenodesis, the opposition of the thumb and index finger with flexion as the tendons are stretched with wrist extension. One should avoid overly stretching the finger flexors initially after injury (“selective tightening”) in C5 and C6 motor level patients to avoid potentially losing the tenodesis action. Tenodesis may allow some individuals with this level of injury to perform activities without splints, that is, feeding. Tenodesis splints can be fabricated but are frequently discarded by patients.
Feeding, grooming, and UE hygiene are usually independent after assistance with setting up the utensils; however, clothing modifications such as Velcro closures on shoes, loops on zippers, and pullover garments are recommended. Assistance for meal preparation and for other homemaking tasks is still required. Transfers may be possible using a transfer board and with loops for LE management, but most often requires assistance. Although persons with a C6 motor level can propel a manual wheelchair with plastic-coated rims, a power wheelchair is often required for long distances, especially if the individual will be returning to the workplace. Power-assist wheels may be of benefit as well. IC may be possible for males after assistance for set-up with assistive devices (
138) but this technique is more difficult for females.
C7, C8 Levels
The C7 motor level adds the elbow extensors (triceps) as the key muscle group; C8 the long finger flexors. The C7 level is considered the key level for becoming independent in most activities at the wheelchair level, including weight shifts, transfers between level surfaces, feeding, grooming, upper body dressing, and light meal preparation (
139). Uneven surface transfers, lower body dressing, and house cleaning may require some assistance. The independent use of a car is possible if the individual can transfer and load/unload their wheelchair.
IC in males can be performed although it is more difficult for females, especially if LE spasticity is present. Surgical options for females including a continent urinary diversion with an umbilical stoma can allow for a cosmetic means to
catheterize easily (
140). Bowel care on a padded commode seat, especially suppository insertion, may still require assistance or the use of adaptive devices (i.e., suppository inserter).
T1-12 Thoracic Levels
Individuals with all levels of paraplegia should be independent with basic ADL including LE dressing, and mobility skills at the wheelchair level on even and uneven surfaces. This includes advanced wheelchair techniques such as curbs, ramps, wheelies (balancing the wheelchair on the two rear wheels), and floor to wheelchair transfers. Bowel and bladder management should be independent.
For most individuals with higher levels of complete thoracic injury, community ambulation is not a functional long-term goal. The lower the level of injury, the greater the trunk control due to abdominal and paraspinal muscle innervation. For lower levels of thoracic injuries, there is improved trunk control to allow for ambulation training with bilateral LE orthoses, as an exercise and for short distance household ambulation, once they have mastered basic wheelchair mobility skills.