CHAPTER 55 Rationale of Minimally Invasive Spine Surgery
Anatomy of the Posterior Paraspinal Muscles
The posterior lumbar paraspinal muscles are part of a larger biomechanical system that includes the abdominal muscles and their fibrous attachment to the spine through the dorsolumbar fascia. This network of muscles is responsible for generating movements of the spine while maintaining its stability.1,2 In addition to maintaining spinal posture in its neutral position, the paraspinal muscles guard the spine from excessive bending that would otherwise endanger the integrity of the intervertebral discs and ligaments.3 Panjabi and colleagues4,5 have proposed that the paraspinal muscles apply minimal resistance inside the neutral zone (NZ) but increase their stiffness exponentially once the range of motion falls outside this NZ. This dynamic stabilizing system is controlled by an interconnected chain of mechanoreceptors embedded in the muscle fascicles, the disc annulus, and the spinal ligaments.6 Functional electromyographic (EMG) studies reveal that spinal stability is achieved by the simultaneous contraction of several agonist-antagonist muscles.3,7,8 Architectural studies suggest that the individual paraspinal muscles may have different primary roles as either movers or stabilizers of the spinal column.9
Multifidus Muscle
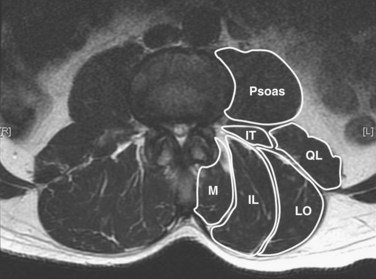
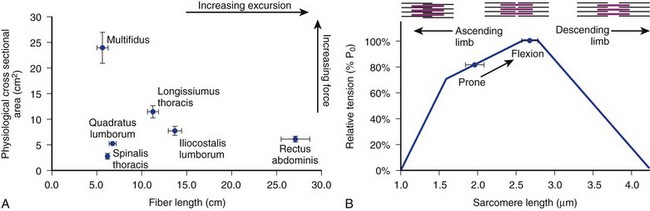
The posterior paraspinal muscles are composed of two muscle groups: (1) the deep paramedian transversospinalis muscle group, which includes the multifidus, interspinales, intertransversarii, and short rotators, and (2) the more superficial and lateral erector spinae muscles that include the longissimus and iliocostalis (Fig. 55–1). These muscles run along the thoracolumbar spine and attach caudally to the sacrum, sacroiliac joint, and iliac wing. The multifidus is the most medial of the major posterior paraspinal muscles and is the largest muscle that spans the lumbosacral junction. It is believed to be the major posterior stabilizing muscle of the spine.3,9,10 Compared with other paraspinal muscles, the multifidus muscle is short and stout. It has a large physiologic cross-sectional area (PCSA) but short fiber lengths. This unique architectural anatomy is designed to create large forces over relatively short distances (Fig. 55–2A).9 Furthermore, the multifidus sarcomere length is positioned on the ascending portion of the length-tension curve (Fig. 55–2B). When our posture changes from standing erect to bending forward, the multifidus can produce more force as the spine flexes forward. This serves to protect the spine at its most vulnerable position.
The multifidus is the only muscle that is attached to both the posterior parts of the L5 and S1 vertebrae and is therefore the sole posterior stabilizer that both originates and inserts to this segment. The morphology of the lumbar multifidus is complex.11 Unlike the other paraspinal muscles that have specific origins and insertions, the multifidus muscle is formed by five separate bands, each having its own origin and several different insertion sites. Each band consists of several fascicles arising from the tip of the spinous process and the lateral surface of the vertebral lamina. Caudally, the different fascicles diverge to separate attachments into the mammillary processes of the caudal vertebrae two to five levels below their origin and downward through each vertebra to the sacrum. For example, fibers from the L1 band insert into the mammillary processes of the L3, L4, and L5 vertebrae to the dorsal part of S1 and then to the posterior superior iliac spine. Biomechanical analyses, based on the multifidus muscle anatomy, show that it produces posterior sagittal rotation of the vertebra, which opposes a counter rotation generated by the abdominal muscles. The multifidus can further increase lumbar spine stability through a ‘bowstring’ mechanism in which the muscle, positioned posterior to the lumbar lordosis, produces compressive forces on the vertebrae interposed between its attachments.12
Erector Spinae Muscles
The erector spinae muscles are composed of the longissimus, iliocostalis, and spinalis (in the thoracic area).11,13 In the lumbar spine, the longissimus is positioned medially and arises from the transverse and accessory processes and inserts caudally into the ventral surface of the posterior superior iliac spine. The laterally positioned iliocostalis arises from the tip of the transverse processes and the adjacent middle layer of thoracolumbar fascia and inserts into the ventral edge of the iliac crest caudally.14 Unilateral contraction of the lumbar erector spinae laterally flexes the vertebral column; bilateral contraction produces extension and posterior rotation of the vertebrae in the sagittal plane. In addition to their role as the major extensor muscles of the trunk, the iliocostalis and the longissimus also exert large compressive loads and lateral and posterior shear forces at the L4 and L5 segments. Although these forces increase the stiffness and stability of the normal vertebral column, the shearing forces may also exacerbate instability and deformity in a malaligned spine.15 In contrast to the multifidus muscle, microarchitectural studies reveal that these muscles are designed as long muscle fascicles with relatively small PCSA. This anatomic morphology suggests that they serve to move the trunk to extension, lateral bending, and rotation. With this type of design, they are less likely to act as primary stabilizers of the vertebral column.16
Interspinales, Intertransversarii, and Short Rotator Muscles
The interspinales, intertransversarii, and short rotator muscles are short flat muscles that lie dorsal to the intertransverse ligament (see Fig. 55–1). The intertransversarii and interspinales run along the intertransverse and interspinous ligaments of each segment. The short rotators originate from the posterior-superior edge of the lower vertebra and attach to the lateral side of the upper vertebral lamina. Because of their small PCSA, they are unable to generate the forces necessary for movement or stability of the spinal column. More likely, they act as proprioceptive sensors rather than force-generating structures.17
Innervation of the Posterior Paraspinal Muscles
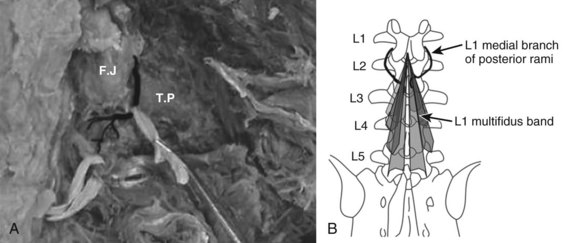
The innervation of all of the posterior paraspinal muscles is derived from the dorsal rami. The iliocostalis is innervated by the lateral branch, while the lumbar fibers of the longissimus receive innervation from the intermediate branch.18 The multifidus is innervated by the medial branch of the dorsal rami (Fig. 55–3). The medial branch curves around the root of the superior articular process and passes between the mammillary and accessory processes to the vertebral lamina, where it branches to supply the multifidus muscle, intertransversarii and interspinales muscles, and zygapophyseal joints.19
During its extramuscular course, the medial branch is strongly attached to the vertebral body in two locations. The first attachment is to the periosteum lateral to the zygapophyseal joints by fibers of the intertransverse ligament. The mamillo-accessory ligament provides the second attachment in the lumbar spine. This strong ligament covers the medial branch and is often calcified.20 These attachments to the vertebra are of clinical importance because they expose the medial branch to possible damage during a midline posterior surgical approach.21
Direct damage to the nerve is also possible during insertion of pedicle screws.22 Insertion of a pedicle screw in the area of the mammillary process can injure the medial branch arising from the cephalad level nerve root, causing denervation injury and consequent atrophy to the multifidus fascicles that arise from the adjacent cephalad level.19,23 For instance, pedicle screws placed at L2 may damage the L1 nerve, which denervates the multifidus bands that originate at L1 and insert into the vertebrae caudally. Moreover, the mono-segmental innervation of the multifidus makes it particularly susceptible to atrophy because it lacks a collateral nerve supply from adjacent muscle segments.24 It is intriguing to consider that dysfunction of this muscle could contribute to adjacent level disc degeneration.
Fiber Type Characteristics of the Paraspinal Muscles
Fiber type analysis can provide important information about the use pattern of a muscle.25,26 There are two major fiber types in skeletal muscles: type I, also known as “slow twitch” and type II or “fast twitch.” The type I fibers possess low ATPase activity, prolonged twitch duration (hence slow twitch), and a low maximal velocity. In addition, type I fibers contain higher mitochondrial content and greater oxidative enzyme complements than type II fibers. Type II fibers are characterized by higher ATPase activities and correspondingly shorter isometric twitch durations. With this design, they are better suited to support the regeneration of ATP through anaerobic mechanisms. Type II fibers can be further subdivided into type IIa and IIx fibers. Type IIx fibers are generally more extreme in each of these respects than the type IIa.26–28
One of the most striking features of the lumbar paraspinal muscles is the predominance of type I muscle fibers compared with other skeletal muscles. Polgar and Johnson studied the distribution of fiber types in 36 human muscles.29,30 A significantly larger type I–to–type II fiber ratio was observed in the multifidus, longissimus, and iliocostalis muscles compared with muscles of the extremities. The predominance of type I fibers and selective type II atrophy have been found in other studies that analyzed fiber type distribution and size in normal paraspinal muscles. It is presumed that along with the adaptation to their stabilizing tonic work characteristics, the phenomenon of type II atrophy can be explained by the sedentary modern life style that deprives these muscles of stimulation from exercise.31 The relatively larger size of type II fibers in professional athletes further supports this assumption.32,33
The morphology of fiber type distribution between the different paraspinal muscles and in different areas inside the muscle is well known. Jorgensen and colleagues34 reported a higher proportion of type I fibers in the longissimus than in the multifidus or iliocostalis muscles. Furthermore, the multifidus muscle is composed of a relatively high percentage of type I fibers, consistent with a postural function. The psoas muscle, on the other hand, is composed of a higher percentage of type II fibers such as in the appendicular muscles.35 Mannion and colleagues36 showed that in women the mean size of the type I fiber is significantly greater than that of either the type IIA or the type IIX, while men have relatively larger-sized type II fibers. In the older population, a loss of muscle mass leads to a decrease in both fiber type sizes with slightly more effect in type II fibers.37–39 The most profound changes in fiber size and fiber type distribution occur in patients with degenerative conditions of the spine.27 Compared with the control, the muscle of low back pain (LBP) patients had a significantly higher proportion of type IIx fiber than type I fibers. They proposed that the relatively low proportion of type I fibers in patients with LBP render them less resistant to fatigue and more susceptible to injury.
Paraspinal Muscle Injury
Characteristics of Paraspinal Muscles in the Postsurgical Spine
Spine surgery inherently causes damage to surrounding muscles.40 This injury can be followed by atrophy of the muscles and subsequent loss of function. Among the different surgical approaches to the spine, it appears that injury to the muscle is greatest when using the midline posterior approach.41 The multifidus muscle is most severely injured when using this approach. Muscle atrophy coincides with decreased muscle cross-sectional area (CSA), which in turn correlates with decreased force production capacity of the muscle.40,42–50
Muscle biopsies obtained from patients undergoing revision spinal surgery exhibit an array of pathologic features that include selective type II fiber atrophy, widespread fiber type grouping (a sign of reinnervation), and “moth-eaten” appearance of muscle fibers.39 Although these pathologic changes can occasionally be found in biopsies from normal individuals, the pathologic changes are more prevalent after surgery.51 Atrophy of the paraspinal muscles can readily be seen in postsurgical back patients.47 Reductions in the CSA of the paraspinal muscles is greatest following a midline approach for a posterolateral fusion.41,48,52
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree