Chapter 137 Prevention of Venous Thromboembolism in Knee Surgery
Limitations of Aspirin and Mechanical Devices
It is estimated that venous thromboembolism (VTE) accounts for up to 160 cases of deep vein thrombosis (DVT), 20 cases of symptomatic nonfatal pulmonary embolism (PE), and 50 cases of fatal PE/100,000/year.24 Deaths from VTE are greater in number than deaths from bowel cancer, prostate cancer, breast cancer, road traffic accidents, or HIV/AIDS.2 PE is the most common preventable cause of hospital death.10 An often overlooked long-term consequence of DVT is the post-thrombotic syndrome, leading to chronic venous insufficiency (CVI) and chronic venous leg ulceration; the incidence is approximately 300 cases/100,000, with approximately 25% being caused by previous DVT that may have been asymptomatic and undiagnosed. Another well-documented debilitating long-term consequence of VTE is chronic thromboembolic pulmonary hypertension.14,26
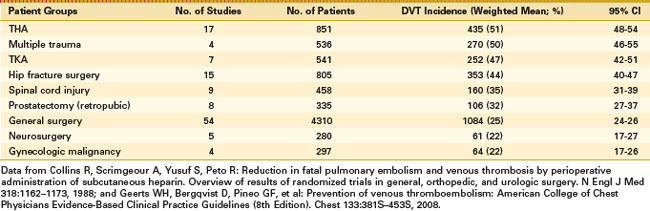
The risk of VTE increases substantially with age10 and DVT occurs in approximately 50% of patients undergoing certain types of surgery if prophylaxis is not used (Table 137-1). Patients undergoing total knee arthroplasty (TKA) without VTE prophylaxis have been shown to have a mean DVT incidence of 47% (95% confidence interval [CI], 42% to 51%), placing them in the highest risk category for postoperative VTE.
In randomized controlled trials to demonstrate the effectiveness of pharmacologic agents for VTE prophylaxis, objective measures are required for the assessment of VTE. The use of mandatory venography has been widely adopted as a surrogate outcome measure to assess the efficacy of anticoagulant drugs. There is skepticism among some clinicians as to whether asymptomatic DVT detected by venography is clinically relevant.5 However, there is a strong correlation between asymptomatic DVT and symptomatic VTE.27 Furthermore, it is important to prevent asymptomatic events to avoid the development of potentially more serious secondary events.
Methods of Prophylaxis
Mechanical Prophylaxis
Graduated Compression Stockings
There is some evidence that graduated compression stockings (GCS) can reduce the incidence of DVT.3 There are fewer studies of GCS compared with those of pharmacologic prophylaxis and these have mostly involved full-length stockings. Although it is anticipated that below-knee stockings should provide a degree of protection against DVT, there are few comparative studies. There are two types of GCS, one for DVT prophylaxis and the other for treatment of CVI. The following are general recommendations for the use of GCS for VTE prophylaxis: (1) they should be worn continuously during the period of immobility to the return of full ambulation; (2) they are contraindicated in patients with critical limb ischemia; (3) they should be measured and fitted for the individual patient; and (4) patient compliance is essential (e.g., ensuring that stockings are not “rolled down”. There is a wide range of available GCS and the choice should take into account their clinical efficacy in providing a pressure of 16 to 20 mm Hg at the ankle, with the patient in a supine position, with graduated compression to the knee or above. Washing and reuse guidelines should be provided and GCS should conform to appropriate manufacturing standards, with independent testing on their compression profile using internationally accepted methods.
Intermittent Pneumatic Compression
Intermittent pneumatic compression (IPC) has also been shown to reduce the incidence of DVT.3 It is more effective than GCS in high-risk patients in combination with anticoagulants or when anticoagulants are contraindicated.
Pharmacologic Prophylaxis
Over the past 30 years, there have been a large number of randomized controlled trials providing indisputable evidence that pharmacologic prophylaxis reduces the risk of VTE. In some early placebo-controlled studies, a reduction in the incidence of fatal PE was noted.10 In subsequent trials, in which one drug was compared with another without a placebo-controlled group, it has been difficult to demonstrate a statistically significant difference in the end point of fatal PE, because its incidence is already low in the comparator drug group being compared with the study drug group. In addition, if an asymptomatic DVT is detected, it will be treated, which will influence the natural history and incidence of PE.
Low-Dose Unfractionated Heparin
Low-dose unfractionated heparin (LDUH) was shown in early studies to reduce the incidence of DVT, symptomatic PE, and fatal PE significantly in patients undergoing major orthopedic surgery.6
Low-Molecular-Weight Heparin
The development of low-molecular-weight heparin (LMWH) led to studies comparing it with LDUH, with meta-analyses of these studies demonstrating its superior efficacy compared with LDUH for the prevention of VTE in orthopedic surgery.16,25
Fondaparinux
In more recent studies, the pentasaccharide fondaparinux, an indirect factor Xa inhibitor, showed superior efficacy for the prevention of VTE compared with the LMWH enoxaparin. However, major bleeding events were more frequent in the fondaparinux group.30
Unfractionated heparin is associated with the rare complication of heparin-induced thrombocytopenia (HIT). LMWHs have a substantially lower risk of HIT compared with unfractionated heparin, but as they are renally excreted, accumulation can occur in patients with significant renal impairment. LMWH should be avoided or used at a lower dose in patients with renal impairment.10 Drug accumulation with fondaparinux may also occur in patients with reduced renal function.
Vitamin K Antagonists
Vitamin K antagonists (VKAs) were until recently the only available oral anticoagulant. A meta-analysis comparing VKAs with alternative prophylaxis has demonstrated that VKAs are significantly less effective than LMWHs for the prevention of DVT, including proximal DVT.23 There was no significant difference in the incidence of PE or death.
Acetylsalicylic Acid
Acetylsalicylic acid (ASA) has been advocated for VTE prophylaxis after major orthopedic surgery.13 However, compared with other available pharmacologic prophylactic agents, it is relatively ineffective. One meta-analysis has shown a benefit for ASA with a reduction in DVT and PE compared with controls, but the reduction was far less than that seen with anticoagulant drugs; other studies have shown no significant benefit with ASA for VTE prophylaxis or have shown that ASA is inferior to other pharmacologic prophylactic agents.10
New Oral Anticoagulants
Recent studies have shown that newer oral anticoagulants may be as effective, or even more effective, than current pharmacologic agents, which are in standard use for VTE prophylaxis.7–9,15,20,31
Dabigatran Etexilate
Dabigatran is an oral direct thrombin inhibitor. Two phase III studies have evaluated dabigatran for the prevention of VTE after TKA. The RE-MOBILIZE11 study randomized 2615 patients to receive dabigatran, 150 mg once daily, dabigatran, 220 mg once daily, or the North American regimen of enoxaparin, 30 mg twice daily for 12 to 15 days. The primary efficacy outcome for dabigatran, 150 and 220 mg, and enoxaparin occurred in 33.7%, 31.1%, and 25.3% of patients, respectively, with dabigatran failing to meet the noninferiority criteria for the primary efficacy outcome compared with enoxaparin. There were numerically less major bleeding events—0.6% for dabigatran compared with 1.4% for enoxaparin—but this was not statistically significant. In the RE-MODEL9 study, the European enoxaparin dosage of 40 mg, once daily for 6 to 10 days, was used and dabigatran was shown to be noninferior compared with enoxaparin. The incidence of major bleeding was similar between the groups.
Rivaroxaban
Rivaroxaban is an oral direct factor Xa inhibitor. There have been four randomized, double-blind, phase III studies (RECORD1, 2, 3, and 4) investigating rivaroxaban for the prevention of VTE in patients undergoing total hip arthroplasty (THA) or TKA.21 A total of 12,729 patients were randomized to receive oral rivaroxaban, 10 mg once daily, starting 6 to 8 hours after surgery or subcutaneous enoxaparin, 40 mg once daily, starting the evening before surgery (RECORD1–3) or 30 mg twice daily, starting 12 to 24 hours after wound closure (RECORD4). RECORD3 recruited patients undergoing TKA for whom the European regimen of enoxaparin was used, whereas patients in RECORD4 received the North American regimen of enoxaparin following TKA. In both RECORD3 and RECORD4, prophylaxis was continued for 10 to 14 days. All patients were followed up for 30 to 35 days after the last dose of study medication.
Apixaban
Apixaban is also an oral direct factor Xa inhibitor evaluated in phase III trials for the prevention of VTE after TKA or THA. In the phase III ADVANCE-1 study in TKA,21 there were 3195 patients randomized to receive apixaban, 2.5 mg twice daily, or enoxaparin, 30 mg twice daily. Both agents were administered 12 to 24 hours postoperatively and continued until days 10 to 14. The primary efficacy outcome of DVT, PE, and all-cause mortality occurred in 9.0% of patients receiving apixaban and 8.8% of patients receiving enoxaparin (P = .06). Rates of major bleeding with apixaban were numerically lower than with enoxaparin (0.7% vs. 1.4%), but this just failed to reach statistical significance. ADVANCE-2 was also in patients undergoing TKA and compared apixaban, 2.5 mg twice daily, with enoxaparin, 40 mg once daily, in 3057 randomized patients. The primary efficacy outcome occurred in 15.1% of patients in the apixaban group and 24.4% in the enoxaparin group, which was statistically significant (P < .001). There was no significant difference in major or clinically relevant nonmajor bleeding (3.5% and 4.8% of patients, respectively).
Rivaroxaban and dabigatran have been shown to have predictable pharmacokinetics and pharmacodynamics17,29 and therefore do not need anticoagulation monitoring, as is required for VKAs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree