Chapter 36 Pregnancy in Women with SLE
Systemic lupus erythematosus (SLE) primarily affects women of childbearing age; pregnancy is therefore a dilemma frequently encountered in this patient population that requires prudent clinical guidance. In the past, because of concerns related to high maternal and fetal mortality rates, medical professionals generally recommended that women with SLE avoid pregnancy. Over the last few decades, however, advances in managing SLE in the context of pregnancy have improved the landscape of risk, and the majority of SLE pregnancies result in a healthy infant and mother.1
Barring previous exposure to cyclophosphamide, women with SLE experience normal fertility rates, and many will become pregnant easily (and sometimes unexpectedly).2 For these reasons, to avoid unwanted or ill-timed pregnancies, addressing contraception with young women with SLE is essential. A small group of women are best advised to avoid pregnancy altogether—those with severe pulmonary hypertension or interstitial lung disease and those with a history of myocardial infarction or arterial thrombosis.
Approximately 4500 pregnancies occur annually in this patient population in the United States.3,4 In some of these patients, pregnancy will lead to a dramatic intensification of symptoms that can be life threatening, but most will experience only a modest increase in symptoms, which may exacerbate the discomforts of pregnancy but will not affect long-term survival.5 For a significant minority, pregnancy is complicated by SLE activity, preterm delivery, preeclampsia, and/or pregnancy loss. With current methods of managing SLE, however, mitigating these risks is possible. Indeed, with careful management and timing of pregnancy, most women with SLE can expect to deliver a child in good health.
Immunobiologic Implications of Pregnancy
Physiologic Alterations of Pregnancy
Pregnancy is heralded by significant physiologic alterations in the mother’s cardiovascular, renal, and immune systems that can have particular bearing on women with SLE. Maternal blood volume increases by 50%, elevating the heart rate, cardiac output, and renal and pulmonary blood flow. Women whose previous disease activity resulted in a damaged cardiopulmonary system may not be able to manage this increased blood volume in pregnancy and may also have particular difficulty with the rapid postpartum loss of volume. Vascular resistance decreases in pregnancy, leading to a mild decrease in blood pressure that may precipitate presyncopal episodes in some women.6
In women who have suffered renal damage, which is not uncommon among women with SLE, escalations in renal blood flow may lead to increased proteinuria. Although a 24-hour urine protein level of 300 mg is considered normal in any pregnancy and less than a two-fold increase in proteinuria is not unexpected, more dramatic levels require immediate attention, because they may signal the onset of either lupus nephritis or preeclampsia.6 In the latter half of pregnancy, alterations in salt concentrations mediated by the kidneys may promote lower extremity edema. For the majority of women, this edema is uncomfortable but not a cause for concern; it can be managed by decreasing salt intake, elevating the legs, and wearing support hose. For some women, however, lower extremity edema may be a symptom of preeclampsia. If it is unilateral, a deep vein thrombosis must be considered.
Even among healthy women, thrombotic risk increases two- to three-fold during pregnancy, which is, in itself, a hypercoagulable condition.7 Women with SLE are at high risk for thrombosis during pregnancy with 1% experiencing deep vein thrombosis, 0.4% experiencing pulmonary embolism, and 0.32% experiencing stroke.4 Moreover, the risk for thrombosis continues for 6 weeks after delivery.
Immunologic Mechanisms of Pregnancy
A significant, yet poorly understood immunologic shift is required to maintain a pregnancy. Because the fetus is an allograft, the maternal immune system must have mechanisms that suppress its typical response to new antigens. The available data suggest that this happens along several routes, including the presentation of unique human leukocyte antigen G (HLA-G) proteins on fetal cells, the production of modified antibodies that selectively bind paternal antigens without stimulating a maternal immune reaction, and increases in the number and activity of regulatory T cells.8,9 How these shifts interact with SLE is unclear. The possibilities vary widely in their implications. The mechanisms that improve maternal-fetal tolerance may also decrease rheumatologic disease; the new antigens might, on the other hand, intensify the production of maternal autoantibodies, or the SLE immune system may be more likely to result in fetal rejection due to impaired tolerance mechanisms. An understanding of these processes is currently too limited to apply them to the management of SLE in pregnancy.
Systemic Lupus Erythematosus Activity in Pregnancy
Types of Systemic Lupus Erythematosus Activity and Their Impact on Pregnancy Outcomes
An estimated 50% of women with SLE will experience a flare during pregnancy.10–17 In most, the flare will be mild, involve the skin or joints, and will not have a major impact on the pregnancy outcome or fetus. Up to 20% of women with SLE will have a more severe flare, involving the kidneys, hematologic disease, serositis, and/or severe arthritis, which can increase the risks for pregnancy loss, preterm birth, and preeclampsia.11,12,15
If SLE is active or platelet counts are low in the first trimester, then the risk for pregnancy loss is increased three- to five-fold (with a 44% rate of pregnancy loss among women with active SLE).18 Women with active SLE are twice as likely to deliver prematurely as a result of medical intervention to protect the health of the mother, preeclampsia, and spontaneous preterm labor.12
Predictors of Systemic Lupus Erythematosus Activity
The three best predictors of SLE activity in pregnancy are the following:
1. Increased SLE activity in the 3 to 6 months before conception
2. Discontinuation of needed immunosuppressive agents during pregnancy
Having minimal lupus activity in the 6 months before becoming pregnant lowers the chances of a significant SLE flare during pregnancy. Women with mild SLE in the 6 months before conception had an 8% risk of increased SLE activity in pregnancy, compared with a 56% risk among women with active SLE in this same period—a more than seven-fold increase.12 Among women with active SLE at the time of conception, a two-fold increase in the risk for a lupus flare exists during pregnancy.11,17 Because SLE activity is a primary cause of preterm birth and pregnancy loss in this population, preventing pregnancy in the months after a significant SLE flare through patient education and a prescription of contraception is important to avoid adverse maternal and fetal outcomes. Women with a history of multiple severe flares are at increased risk for flares in pregnancy. In addition, discontinuation of hydroxychloroquine (HCQ) before or during pregnancy increases the risk for SLE flares.13,19
To decrease the risk of SLE activity in pregnancy requires not only careful planning but also a willingness to modify, yet continue, immunosuppressive therapy. For women with a history of a significant flare, particularly lupus nephritis or significant cytopenias, the discontinuation of immunosuppression for pregnancy may be counterproductive. Although cyclophosphamide and mycophenolate mofetil (MMF)—and probably mycophenolic acid, although no published data is available—pose significant risks for pregnancy loss and for teratogenic effects on the fetus, other immunosuppressants are much safer. Azathioprine has been well documented in large studies of women with solid organ transplants and inflammatory bowel disease and has been determined to be safe and to have limited, if any, teratogenic risk.20
Pregnancy Outcomes in Systemic Lupus Erythematosus with Mediators of Complications
Whether or not SLE is active, women with SLE often have complicated pregnancies: One third will result in a cesarean section, another one third will result in preterm birth, and up to 20% will be affected by preeclampsia.21–23 Offspring outcomes are generally positive, although low birth weight and small-for-gestational-age (SGA) status are not uncommon. Rates of maternal mortality do not appear to exceed those of women with SLE who are not pregnant. Although an SLE pregnancy can present a range of challenges requiring careful medical management, overall outcomes are good even when complications arise.
Pregnancy Loss
Approximately 20% of SLE pregnancies result in miscarriage or stillbirth.1 Although the risk of miscarriage, which, by definition, occurs before 20 weeks’ gestation, is not significantly elevated in patients with SLE relative to that of the general population, the risk of stillbirth, which occurs after 20 weeks’ gestation, is elevated by approximately eight-fold to 5% to 10% of pregnancies, according to several studies12,23 (Table 36-1).
TABLE 36-1 Pregnancy Outcomes in Prospective Cohorts of Pregnancies in Women with Systemic Lupus Erythematosus
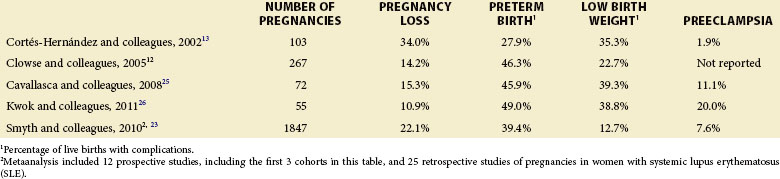
Increased lupus activity and antiphospholipid syndrome (APS) appear to be the two most important predictors of pregnancy loss. Among a Greek cohort, fetal loss occurred in 75% of pregnancies of women with highly active SLE, compared with 14% of pregnancies in those without active lupus and 5% of non-SLE pregnancies.24 Although lupus activity did not affect rates of miscarriage, it resulted in a three-fold increase in the stillbirth rate in the Hopkins lupus pregnancy cohort study.12 Careful consideration must also be given to the timing of SLE activity; it directly correlates with the rates of pregnancy loss, with early pregnancy activity presenting the greatest cause for concern. When lupus activity is present at the time of conception and in the first trimester, the risk of pregnancy loss, particularly stillbirth, increases by up to three-fold.5 Moreover, first-trimester proteinuria, thrombocytopenia, and hypertension each represent an independent risk factor for pregnancy loss, introducing a 30% to 40% chance of pregnancy loss.18 Managing disease activity is therefore essential to achieve clinical remission and thus reduce the risk of fetal loss.
Preterm Birth
Women with lupus are more likely to deliver prematurely, before 37 weeks’ gestation. In one population-based study,27 preterm deliveries occurred at a rate of 21% for women with SLE, almost six-fold higher than in healthy women. Cohorts at tertiary referral centers, however, suggest a more dramatic risk, with rates ranging from 20% to 54%.10–17,21,28 Again, active SLE during pregnancy is the primary risk factor.12 Although the rate of preterm birth is estimated to be 33% in women with quiescent SLE, it dramatically increases to 66% in pregnant women with an SLE flare.21 Other risk factors for preterm birth include lupus activity in the months before pregnancy, higher prednisone doses, and hypertension.
Although a substantial proportion of early deliveries are medically induced to preserve maternal and fetal health (e.g., as in the context of preeclampsia), the majority of early deliveries result from spontaneous processes.14,21 A prominent immediate cause of preterm birth among patients with lupus is the premature rupture of membranes (PROM).14,29 In pregnant women without SLE, approximately one third of spontaneous preterm births are associated with chorioamnionitis, an infection in the uterus. The inflammation associated with chorioamnionitis leads to the dissolution of the fetal membranes, a ripening of the cervix, and uterine contractions, all of which induce premature delivery. At this time, no data have been published concerning the rate of chorioamnionitis in SLE pregnancies, but placenta studies have not demonstrated increased rates of infection or pathologic abnormalities.30 Although the inflammation typical of active lupus may affect the uteroplacental unit in ways similar to chorioamnionitis, research has yet to clarify the role of inflammation in preterm birth.
Preeclampsia
Women with SLE are at particularly high risk for developing preeclampsia in pregnancy. Although preeclampsia affects 5% to 8% of all pregnancies in the United States, it is far more common in SLE pregnancies with an estimated 7% to 35% rate of occurrence.3,11,23,31,32 According to research, not only will an average of one in four women with SLE develop preeclampsia, but the risks are even greater for women with preexisting hypertension or a history of lupus nephritis.3,4,33 Other risks include first pregnancy, a history of preeclampsia, active SLE at conception, positive anti–double stranded DNA (anti-dsDNA) or antiribonucleoprotein antibodies, low complement levels, and obesity.11,14,31,32
The cause of preeclampsia remains under investigation. Preeclampsia is generally thought to arise from vascular dysfunction in the placenta, possibly the result of poor implantation and diminished trophoblast invasion of the uterine spiral arteries.34 Several experimental markers for preeclampsia, including soluble Fms-like tyrosine kinase 1 (sFlt-1) and placental growth factor (PGF), have been found to correspond to preeclampsia in patients with lupus, as they do in women without SLE.35
Daily low-dose aspirin may decrease the risk for preeclampsia, premature delivery, and fetal loss, especially among those already at high risk for such complications. Aspirin minimizes two factors that contribute to preeclampsia: (1) the vasoconstrictor thromboxane and (2) platelet activation. In a Cochrane Review36 of aspirin in pregnancy, which included 57 trials, none of which specifically enrolled patients with autoimmune disease, and over 37,000 women, low-dose aspirin was found to be safe and even potentially beneficial for both mother and baby. For women at high risk for preeclampsia, daily low-dose aspirin can decrease the risk for preeclampsia by 25% and the risk for pregnancy loss by 31%.36 Considering the particularly high risk for preeclampsia in SLE pregnancies, 81 mg of aspirin per day should be considered for all pregnant women with lupus.
Offspring Outcomes
Preterm birth is perhaps the greatest risk faced by offspring of mothers with SLE, because infants born before 28 weeks’ gestation are more likely to endure long-term medical complications or die soon after birth. SLE activity during pregnancy greatly increases the probability of a dangerously early delivery. In the Hopkins lupus pregnancy cohort,12 delivery between 24 and 28 weeks’ gestation occurred in 17% of all pregnancies with active SLE, whereas delivery during this high-risk period occurred in only 6% of those pregnancies in which SLE was quiescent.
Whether low birth weight is a higher risk for offspring of women with SLE than it is in women without SLE is still a matter of debate. Because high rates of preterm birth complicate any study of birth weight, especially among lupus pregnancies, weight is generally corrected according to gestational age. Infants who weigh less than the tenth percentile based on national norms are considered SGA. On average, of all SLE pregnancy cohort births, 9.4% were SGA, which is comparable to expectations in the general population.21 However, certain cohorts had SGA rates as high as 35%.12,14
Because the risk for SGA is relatively low, clear risk factors have not been identified. Placental insufficiency could lead to slow fetal growth and inadequate weight gain. Accordingly, with placental studies reporting a higher incidence of thrombosis among SLE pregnancies, some of these pregnancies can be expected to produce growth-restricted infants.30
Maternal Mortality Rate
The maternal mortality rate for women with SLE—325 per 100,000 pregnancies—is estimated to be twenty-fold higher than it is for average women.4 However, when the annual death rate for all women with SLE is taken into consideration—approximately 1000 per 100,000 patient years—it appears that pregnancy probably does not increase the risk of death for women with SLE.37 However, women with SLE who have had previous arterial thrombosis, a weakened heart from myocarditis, previous myocardial infarction or valve disease, uncontrolled hypertension, pulmonary hypertension, or a previous severe SLE flare during pregnancy may be placed at a higher risk for death by becoming pregnant.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








