Disuse Atrophy
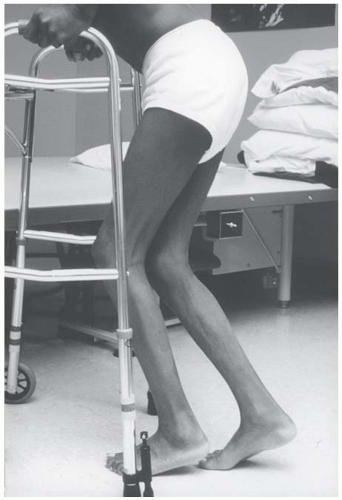
Decrease in the size of muscle fibers and reduction of muscle mass are the hallmark of muscle atrophy. In a lower motor neuron lesion, the atrophy is regional and related to the particular nerve or root. Atrophy associated with muscle disease is more pronounced in the proximal muscles. The atrophy of disuse is generalized or localized to the immobilized limb(s) and more prominent in the antigravity muscles. It is a consequence of the limited physical activity and musculoskeletal loading that occurs during immobilization, immobility, bed rest, or exposure to microgravity during space flight. As a general rule, increased muscular activity leads to hypertrophy, whereas limited physical activity leads to disuse atrophy and weakness. The changes in muscle fiber size either in atrophy or in hypertrophy and phenotype changes of muscle fibers are the results of remarkable muscle plasticity and adaptability to external physical demands to generate adequate or peak contraction and endurance.
Disuse atrophy is defined as an alteration of metabolism and muscle cell homeostasis in response to muscle inactivity. Recent studies indicate that muscle protein synthesis as well as whole body protein production is significantly reduced during immobility and is considered the main contributor to muscle atrophy. The rate of muscle wasting during bed rest is slow during the first 2 days but becomes rapid thereafter. By 10 days, it reaches 50% of eventual muscle weight loss. Similarly, muscle protein synthesis is reduced to 50% of the baseline level at 14 days of immobilization and then gradually tapers off to reach a new steady state (
6). Reeves et al. (
7) studied skeletal muscle changes in healthy men exposed to microgravity condition and found a decrease in calf muscle volume by 29%, resting fascicle length of medial head of gastrocnemius and their pennation angulation by 10% and 13%, respectively, and physiological cross-sectional area (PCSA) by 22%. In disuse atrophy, especially if muscle is immobilized in a shortened position, that is flexion, the number of sarcomeres in series is reduced as a result of diminished chronic stretch and adaptation of the muscle to a new resting length. By contrast, immobilization in an elongated muscle resting position or during musculoskeletal growth increases the number of sarcomeres in series (
8). In addition, the number of sarcomeres in parallel is reduced contributing to the reduction of muscle fiber PCSA (muscle volume/fascicle length) (
9). From data obtained on the
PCSA, the reduction in the number of sarcomeres in parallel is twofold greater than reduction of sarcomeres in series. This indicates that reduction of isometric strength is in proportion to the loss of total number of sarcomeres in parallel and that they are more affected by disuse (
9).
Disuse atrophy of type I and type IIa muscle fibers is more prominent than type IIb fiber atrophy during immobility. The mean size of type I fibers in human soleus muscle is decreased by 12% and 39% at 2 and 4 months of bed rest, respectively. This reduction could be prevented by simulating gravity loading for 10 hours a day in supine position (
10). Individual muscle thickness can be clinically measured in vivo for some muscle by ultrasound scanning. Using this technique, the reduction of muscle thickness after 20-day bed rest is on average -2.1% to -4.4% and varies significantly for different muscles of the lower limbs (
11). Magnetic resonance imaging (MRI) studies have, however, revealed much greater percentages of atrophy of the muscles in the lower limbs with the same duration of bed rest (
12). Furthermore, consecutive MRI studies of thigh and calf muscles revealed that cross-sectional area and muscle volume of the gastrocnemius and soleus muscles were reduced to a greater extent than knee extensors and flexors (-9.4% to -10.3% vs. -5.1% to -8.0%) after 20 days of bed rest (
12). However, using specific tests, the mean cross-sectional area reduction for the dark adenosine triphosphatase (ATPase) and light ATPase fibers is 46% and 69%, respectively, after 20 days of immobility (
13).
Along with muscle fiber atrophy, the synthesis of collagen fibers is also reduced, although this reduction is much less than the reduction in synthesis of muscle proteins. This leads to a relative increase of muscle collagen content and changes in its mechanical elastic properties. The increase in muscle stiffness and alteration in viscoelastic properties of plantar flexors during space flight correlated with the duration of the flight, but the changes were less prominent than during bed rest (
14). In both situations, weight bearing is limited, but joint motion is free and abundant during space flights. In healthy subjects with normal mobility, the main resistance to excessive elongation of muscle fiber is due to resistance of the myofibrils and in a lesser part to the sarcolemma itself. Titin, a myofibrillar protein, appears to have a major role in providing resistance to passive elongation, and it is increased in immobility. When titin is chemically removed, this resistance to passive stretch is significantly diminished (
14).
Prominent histochemical changes also occur in muscle during bed rest and immobility. Serum creatine kinase (CK) isomer and fibroblast growth factor release (after myofiber injury) are both reduced during bed rest, and this is proportional to the reduction in muscle fiber size. Resistance exercises during bed rest significantly increase the level of these factors and prevent muscle fiber atrophy, indicating that myofiber wound-mediated fibroblast growth factor may play an important role in disuse atrophy (
15). Myostatin is a growth factor-beta protein that inhibits muscle synthesis and is increased during bed rest. After 25 days of bed rest, the total lean body mass declines by an average of 2.2 kg, and plasma myostatin-immunoreactive protein level increases by 12%. It is speculated that suppression of myostatin may prevent muscle atrophy during space flight (
16).
The muscle mass loss associated with aging is called sarcopenia. It is a major cause of disability and frailty in the elderly population. Inactivity is one of the many factors responsible for development of sarcopenia, along with decline in number of alpha-motor neurons, reduction in growth hormone, inadequate protein intake, and chronic overproduction of catabolic cytokines. High-intensity resistance exercise can reverse sarcopenia, indicating that physical inactivity is a major risk factor for weakness in elders (
17). Many articles have been written about functional decline in hospitalized and nursing-home patients. General deconditioning in elderly persons is a frequent cause of functional decline, falls, and increased dependency. Reconditioning takes much longer in the elderly than in younger persons.
Although the primary reason for atrophy is reduction of muscle protein synthesis, an increased protein breakdown with nitrogen loss is also found during immobility despite the fact that the major energy sources during bed rest are primarily derived from carbohydrates and fat. It is believed that decline of mitochondrial function and the reduction of protein synthesis are the main reasons for the onset and progression of disuse atrophy. However, in the later stages of disuse muscle atrophy, the process of protein degradation may become more prominent than decrease in protein synthesis. This may be aggravated by gastrointestinal mechanisms, such as loss of appetite and reduced intestinal absorption of protein. Although daily loss of nitrogen for an immobilized healthy person may reach 2 g/day, a nutritionally depleted person may lose as much as 12 g/day. Urinary excretion of creatine is minimal under normal conditions, except in pregnancy and infancy. The excretion of creatine is much greater in starvation, diabetes, muscular dystrophy, hyperthyroidism, and fever, as well as during immobility. Prolonged bed rest and weightlessness causes a significant increase in the excretion of both creatine and creatinine, the mechanism which is not well understood (
18).
Loss of Strength
Muscle weakness, reduced endurance, and tolerance to work are the functional consequences of muscle atrophy. The maximal strength of a muscle can fall to 25% to 40% of baseline level when a person is exposed to minimal exertions over a 2- or 3-week period (
19). During strict bed rest, muscles may lose 10% to 15% of their original strength per week and, over 4 weeks, 35% to 50%. The loss of strength is rapid after the first day of immobilization and reaches its maximum 10 to 14 days later (
20). Resistance leg exercises performed above 50% of maximum every second day, for 20 minutes by healthy subjects on bed rest, can maintain the muscle protein synthesis at the same level as healthy subjects engaged in normal physical activity (
21). A study on dynamic leg press training during bed rest demonstrated preservation of baseline cross-sectional area and strength for the knee extensors and flexors but not for ankle plantar flexors and ankle dorsiflexors. This can adversely affect functional locomotion, as ankle plantar flexion contributes to forward propulsion (
22).
The loss of strength associated with disuse atrophy is more prominent in the lower limbs than in the upper limbs. Loss of muscle power during immobilization reaches -20% to -44% in knee flexors and extensors, comparing to an insignificant loss in the upper limbs of -5%. The decrease in peak muscle tension of knee flexors and extensor ranges from -19% to -26%, far more than the reduction in cross-sectional area of these muscles (-7%), indicating that loss of strength and power is proportionally greater than reduction in size of the respective muscles (
23). The major contributors to the loss of strength and endurance in persons with disuse atrophy are the reduced number of myofibrils per fiber volume, reduction in size and number of mitochondria, muscle fiber nuclei, and reduction of sarcomeres in parallel (
10).
Another aspect of disuse weakness is reduction of maximal instantaneous muscular power. It can be reduced to a greater extent than the peak muscle strength of one repetition maximum in bed rest subjects. The loss of strength has been well documented in immobility; however, the loss of explosive muscle power as measured by the maximal jump with both feet on force plate was only recently studied. After 45 days of bed rest, instantaneous muscular power was reduced 24%, and recovery required one and half months of remobilization (
24). These findings indicate that specific training for instantaneous power should be considered during space flight or prolonged bed rest to preserve functions such as standing up, climbing, and performing transfer functions.
Decline in muscle twitch and tetanic tension parallels the decline in muscle strength. In rats, maximal tension obtained by electrical stimulation in the soleus muscle declined significantly after 6 weeks of immobilization (
25). Changes in contractile forces of immobilized muscle are the result of diminished levels of myofibrillar proteins and also due to a reduction of sarcoplasmic reticulum calcium ion uptake, but not the rate of its release (
26).
Loss of Endurance
Multiple studies have demonstrated that prolonged inactivity causes a significant and progressive reduction in muscle endurance. Unexercised muscle demonstrates a reduction of adenosine triphosphate (ATP) and glycogen storage sites and rapid depletion of them after resumption of activity. Reduction of muscle protein synthesis and oxidative enzyme function, and premature anaerobic energy production with rapid accumulation of lactic acid, are important factors leading to fatigability and reduced endurance (
27).
Metabolic and enzymatic alterations in unexercised muscle result from reduced demand for oxygen and reduction in the blood supply. Initially after bed rest, succinate dehydrogenase enzyme activity per muscle fiber increases, but later, the overall amount is reduced (
28). Oxidative enzyme activity and content as well as the number and size of mitochondria are all reduced during immobility (
29). Unexercised muscle also shows a decreased ability to utilize fatty acids when compared with trained subjects.
Ferretti et al. studied peripheral and central factors that contribute to the decline of VO
2max after 42 days of bed rest. VO
2max was found reduced by 16%, cardiac output by 30%, and oxygen delivery by 40%, which parallels the reduction in muscle cross-sectional area of 17%, volume density of mitochondria by 16%, and total mitochondria volume in the magnitude of 28%. Oxidative enzyme activity falls by 11%. These decrements indicate a significant contribution of both peripheral and central factors in causing the decline in VO
2max after immobility, confirming a close interrelationship between the muscular and the cardiovascular system (
27).
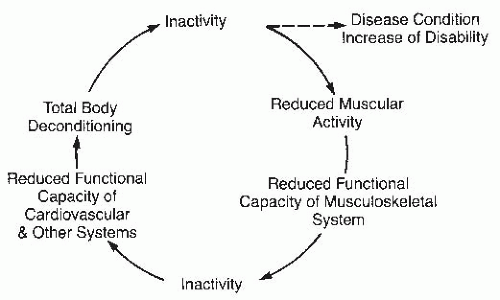
The following sequence of events transpires in the deconditioning process. Prolonged reduction of muscle repetitive contractions below 50% of maximum alters muscle protein synthesis and decreases glycogen and ATP storage, causing a reduction of oxidative enzymes, mitochondrial function, and microvascular circulation, impacting muscle metabolic activity. As a result, the oxygen supply is attenuated, and the extraction of oxygen from blood is diminished, further negatively affecting VO
2max and cardiovascular reserve. The loss of muscle mass leads to reduction of muscle strength and endurance, reducing muscle blood flow, red blood cell delivery, oxidative enzyme activity, and oxygen utilization in the muscle precipitating a further loss of musculoskeletal and cardiovascular functional reserve to low or dangerous levels. In this cascade of events, specific muscle gene activation and expression are altered as well. Physical inactivity causes change in muscle fiber type composition and decreases formation of oxidative muscle fiber types I and IIa, the main factors in reduction of endurance and fitness (
30).