Perioperative Pain Management
Terese T. Horlocker and Sandra L. Kopp
Key Points
• Lumbar plexus blockade is superior to neuraxial analgesia for patients undergoing major hip surgery.
• Psoas compartment block provides anesthesia/analgesia to the complete lumbar plexus.
Introduction
Pain after major hip surgery is severe. Failure to provide adequate analgesia impedes aggressive physical therapy and rehabilitation and potentially delays hospital dismissal. Traditionally, postoperative analgesia following total joint replacement was provided by intravenous patient-controlled analgesia (PCA) or epidural analgesia. However, each technique has distinct advantages and disadvantages. For example, opioids do not consistently provide adequate pain relief and often cause sedation, constipation, nausea/vomiting, and pruritus. Epidural infusions containing local anesthetics (with or without an opioid) provide superior analgesia but are associated with hypotension, urinary retention, motor block–limiting ambulation, and spinal hematoma secondary to anticoagulation.1 Single-dose and continuous peripheral nerve techniques that block the lumbar plexus (fascia iliaca, femoral, psoas compartment blocks) with/without sciatic nerve blockade have been used with success for total hip replacement patients.1–4 Appreciation of the indications, benefits, and side effects associated with both conventional and novel analgesic approaches is paramount in maximizing rehabilitative efforts and improving patient satisfaction. This chapter will discuss the analgesic techniques unique to patients undergoing hip surgery, with a focus on those undergoing primary total or revision total hip arthroplasty.
Multimodal Analgesia
Multimodal analgesia is a multidisciplinary approach to pain management, with the aim of maximizing the positive aspects of treatment while limiting associated side effects. Because many of the negative side effects of analgesic therapy are opioid related (and dose dependent), limiting perioperative opioid use is a major principle of multimodal analgesia. Anti-inflammatory medications and acetaminophen are valuable adjuvants to systemic opioids. The addition of nonopioid analgesics reduces opioid use, improves analgesia, and decreases opioid-related side effects. The use of peripheral or neuraxial regional anesthetic techniques and a combination of opioid and nonopioid analgesic agents for breakthrough pain results in superior pain control, attenuation of the stress response, and decreased opioid requirements.
Systemic Analgesics
Opioid Analgesics
Adequate analgesia achieved with systemic opioids is frequently associated with side effects, including sedation, nausea, and pruritus. However, despite these well-defined side effects, opioid analgesics remain an integral component of postoperative pain relief. Systemic opioids may be administered by intravenous, intramuscular, and oral routes. Current analgesic regimens typically employ intravenous PCA for 24 to 48 hours postoperatively, with subsequent conversion to oral agents. The PCA device may be programmed for several variables, including bolus dose, lockout interval, and background infusion (Table 27-1). The optimal bolus dose is determined by the relative potency of the opioid; insufficient dosing results in inadequate analgesia, whereas excessive dosing increases the potential for side effects, including respiratory depression. Likewise, the lockout interval is based on the onset of analgesic effects; too short of a lockout interval allows the patient to self-administer additional medication before achieving the full analgesic effect (and may result in accumulation/overdose of the opioid). A prolonged lockout interval will not allow adequate analgesia. The optimal bolus dose and lockout interval are not known, but ranges have been determined. Varying settings within these ranges appears to have little effect on analgesia or side effects. Although most PCA devices allow the addition of a background infusion, routine use in adult opioid-naive patients is not recommended; however, a background opioid infusion may have a role in the treatment of opioid-tolerant patients. Because of variation in patient pain tolerance, PCA dosing regimens may have to be adjusted to maximize the benefits and minimize the incidence of side effects.
Table 27-1
Intravenous Opioids for Patient-Controlled Analgesia
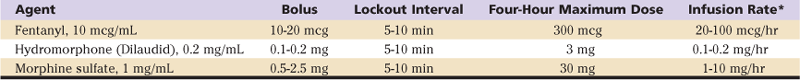
*A background infusion rate is not recommended for opioid-naive patients.
From Lennon RL, Horlocker T: Mayo Clinic analgesic pathway: peripheral nerve blockade for major orthopedic surgery, Rochester, Minn, 2006, Mayo Clinic Scientific Press, Table 1, p 109, with permission.
Adverse effects of opioid administration can cause serious complications in patients undergoing major orthopedic procedures. In a systematic review, Wheeler and associates5 reported gastrointestinal issues (nausea, vomiting, ileus) in 37%, cognitive effects (somnolence and dizziness) in 34%, pruritus in 15%, urinary retention in 16%, and respiratory depression in 2% of patients receiving PCA opioid analgesia.
Oral opioids (Table 27-2) are available in immediate-release and controlled-release formulations. Although immediate-release oral opioids are effective in relieving moderate to severe pain, they must be administered as often as every 4 hours. When these medications are prescribed “as needed” (prn), there may be a delay in administration and a subsequent increase in pain. Furthermore, interruption of the dosing schedule, particularly during the night, may lead to an increase in the patient’s pain. Adverse effects of oral opioid administration are considerably less compared with those of intravenous administration and are mainly gastrointestinal in nature.5
Table 27-2
Oral Analgesics
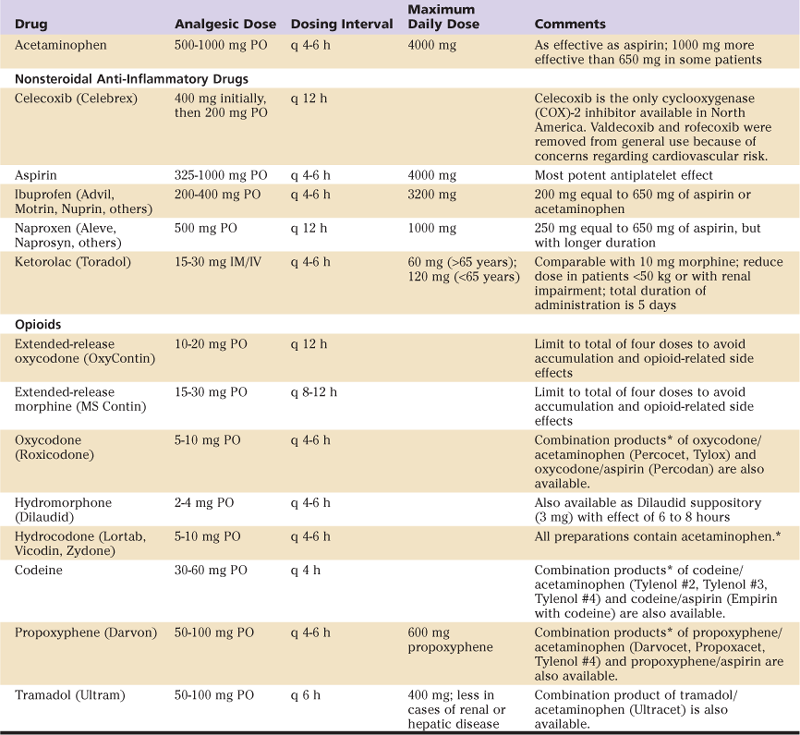
IM, Intramuscularly; IV, intravenously; PO, orally.
*Dose in combination products limited by total acetaminophen or aspirin ingestion.54
From Lennon RL, Horlocker T: Mayo Clinic analgesic pathway: peripheral nerve blockade for major orthopedic surgery, Rochester, Minn, 2006, Mayo Clinic Scientific Press, Table 2, pp 110–111, with permission.
A controlled-release formulation of oxycodone (OxyContin) has been shown to provide therapeutic opioid concentrations and sustained pain relief over an extended time period. Combined with prn oxycodone for breakthrough pain, scheduled administration of controlled-release oxycodone maximizes the analgesia and decreases associated side effects. However, because pain decreases substantially over the first 24 to 36 hours, sustained-release formulations should be limited to the early postoperative period in most cases.
Tramadol (Ultram) is a centrally acting analgesic that is structurally related to morphine and codeine (but is not truly an opioid). Its analgesic effect is manifest through binding to the opioid receptors and blocking the reuptake of both norepinephrine and serotonin. Tramadol should be used with caution in patients taking certain antidepressant medications (e.g., selective serotonin reuptake inhibitors) affecting the levels of these two neurotransmitters. Tramadol has gained popularity because of the low incidence of adverse effects, specifically, respiratory depression, constipation, and abuse potential. Thus, tramadol may be used as an alternative to opioids in a multimodal approach to postoperative pain, specifically in patients who are intolerant to opioid analgesics.
Nonopioid Analgesics (Acetaminophen and Nonsteroidal Anti-Inflammatory Drugs)
The addition of nonopioid analgesics reduces opioid use, improves analgesia, and decreases opioid-related side effects. The multimodal effect is maximized through selection of analgesics that have complementary sites of action. For example, acetaminophen acts predominantly centrally, while other nonsteroidal anti-inflammatory drugs (NSAIDs) exert their effects peripherally.
The mechanism of analgesic action of acetaminophen has not been fully determined. Acetaminophen may act predominantly by inhibiting prostaglandin synthesis in the central nervous system. Acetaminophen has very few adverse effects and is an important addition to the multimodal postoperative pain regimen, although the total daily dose must be limited to 4000 mg. It is important to note that many oral analgesics are an opioid-acetaminophen combination. In these preparations, the total dose of opioid will be restricted to the acetaminophen ingested.
NSAIDs have a mechanism of action through the cyclooxygenase (COX) enzymatic pathway and ultimately block two individual prostaglandin pathways. The COX-1 pathway is involved in prostaglandin E2–mediated gastric mucosal protection and thromboxane effects on coagulation. The inducible COX-2 pathway is mainly involved in the generation of prostaglandins included in the modulation of pain and fever but has no effect on platelet function or the coagulation system. In general, NSAIDs block both COX-1 and COX-2 pathways. Traditionally, NSAIDs have been viewed as peripherally acting agents. However, a central analgesic effect may occur through inhibition of spinal COX.
The introduction of specific COX-2 inhibitors represented a breakthrough in the treatment of pain and inflammation. However, despite their efficacy, two (rofecoxib [Vioxx]; valdecoxib [Bextra]) of three COX-2 inhibitors were voluntarily removed from general use because of an increased relative risk for confirmed cardiovascular events, such as heart attack and stroke, after 18 months of treatment. Celecoxib (Celebrex) is currently the only COX-2 inhibitor available in the United States, although the Food and Drug Administration (FDA) has requested that safety information be included regarding potential cardiovascular and gastrointestinal risks of all selective and nonselective NSAIDs except aspirin.*
Although numerous NSAIDs have been used in the perioperative management of pain, ketorolac is the only NSAID that can be given parenterally. An intravenous dose of ketorolac 10 to 30 mg was found to have similar efficacy to 10 to 12 mg of intravenous morphine. In surgical patients, ketorolac reduces opioid consumption by 36%. Because of the potential for serious side effects, ketorolac should be used for 5 or fewer days in the adult population with moderate to severe acute pain.6
Major side effects limiting NSAID use to postoperative pain control (renal failure, platelet dysfunction, and gastric ulcers or bleeding) are related to nonspecific inhibition of the COX-1 enzyme.6 Advantages of COX-2 inhibitors include lack of platelet inhibition and a decreased incidence of gastrointestinal effects. All NSAIDs have the potential to cause serious renal impairment. Inhibition of the COX enzyme may have only minor effects in the healthy kidney, but unfortunately can lead to serious side effects in elderly patients and those with a low-volume condition (blood loss, dehydration, cirrhosis, or heart failure). Therefore, NSAIDs should be used cautiously in patients with underlying renal dysfunction, specifically in the setting of volume depletion due to blood loss.6 Similar to the COX-2 inhibitors, NSAIDs interfere with the inhibitory COX-1 effect of aspirin on platelet activity and may counter its cardioprotective effects.7
The effects of NSAIDs on bone formation and healing are of concern to the orthopedic population. Although data are conflicting, evidence from animal studies suggests that COX-2 inhibitors may inhibit bone healing.8 Thus, adverse effects of COX-2 inhibitors must be weighed against the benefits. Until definitive human trials are performed, it is reasonable to be cautious with the use of COX-2 inhibitors, especially when bone healing is critical.
Neuraxial Analgesia
A variety of single-dose and continuous infusion neuraxial techniques may be performed to provide analgesia following major hip surgery. A single dose of neuraxial opioid may be efficacious as a sole analgesic agent for moderate pain of limited duration, such as that associated with primary hip arthroplasty.9 However, the prolonged moderate to severe pain associated with revision arthroplasty typically necessitates supplemental oral or intravenous analgesic agents or a continuous neuraxial infusion.
Single-Dose Spinal and Epidural Opioids
Neuraxial opioids provide superior analgesia compared with systemic opioids. The onset and duration of neuraxial opioids are determined by the lipophilicity of the drug. For example, lipophilic opioids, such as fentanyl, provide a rapid onset of analgesia, limited spread within the cerebrospinal fluid (and less respiratory depression), and rapid clearance/resolution. Conversely, hydrophilic opioids, including morphine and hydromorphone, have a longer duration of action but are associated with a higher frequency of side effects such as pruritus, nausea and vomiting, and delayed respiratory depression (Table 27-3). A new sustained-release formulation of epidural morphine (Depodur) has been released. Limited information exists regarding its efficacy following orthopedic surgery.10 The analgesic effect is present for approximately 48 hours. Unfortunately, Depodur is not to be administered in the presence of local anesthetics (i.e., an epidural anesthetic may not be converted to provide epidural analgesia). It is important to note that the central side effects of opioid administration are much more common (and more prolonged) following neuraxial administration than with all other routes. For example, in a large series, the frequency of pruritus, nausea and vomiting, and respiratory depression was 37%, 25%, and 3% with an intrathecal morphine injection.11 Therefore, patients who exhibit sensitivity to an opioid when administered systemically should not receive that opioid neuraxially.
Table 27-3
Dosing Regimens for Neuraxial Opioids*
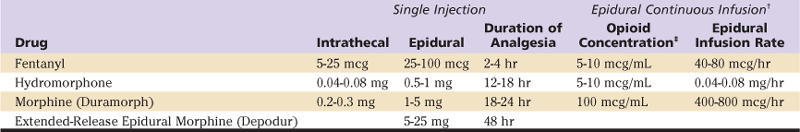
*Note units vary across agents for single dosing (mcg, mg).
Epidural Analgesia
Epidural analgesia may consist of an opioid, a local anesthetic, or a combination local anesthetic–opioid infusion (see Table 27-3). The combination of a local anesthetic–opioid creates a synergistic analgesic effect and allows a lower concentration of each component of the solution. For example, without an opioid adjuvant, the concentration of a local anesthetic solution may be sufficiently high to result in a dense sensory and motor block; the patient may be unable to ambulate or void.12 Likewise, a pure opioid epidural infusion may not provide adequate analgesia.13 As a result, most epidural solutions consist of dilute concentrations of both local anesthetic and opioid. Use of this combination results in superior analgesia, minimal sensory and motor block (allowing ambulation and mobilization), and a decreased incidence of opioid-related side effects (nausea/vomiting and pruritus). Although epidural analgesia provides excellent analgesia, the associated risk of spinal hematoma in (anticoagulated) patients with indwelling epidural catheters led to a search for alternative methods of providing postoperative analgesia following major orthopedic surgery.
Peripheral Regional Anesthetic Techniques
Lower extremity peripheral techniques, which allow complete unilateral blockade, have traditionally been underutilized.14 In part, this is a result of the widespread acceptance and safety of spinal and epidural anesthesia. Furthermore, unlike the brachial plexus, nerves supplying the lower extremity are not anatomically clustered, where they can be easily blocked with a relatively superficial injection of local anesthetic. Because of anatomic considerations, lower extremity blocks are technically more difficult and require more training and practice before expertise is acquired. Many of these blocks were classically performed using paresthesia, loss of resistance, or field block technique; success was variable. Advances in needles, catheters, and nerve stimulator technology have facilitated localization of neural structures and have improved success rates. These blocks are safe and have certain advantages, such as postoperative pain relief and lack of complete sympathectomy, which make them ideal for selected patients. Although single-injection techniques have been used, the duration of effect is not sufficient to result in major improvements in analgesia or outcome.15,16
Over the past decade, applications have focused on prolonged postoperative analgesia (with an indwelling catheter) to assist rehabilitation and hospital dismissal.2,3,17,18 Several studies have demonstrated that unilateral peripheral block provided a quality of analgesia and surgical outcomes similar to those of continuous epidural analgesia, but with fewer side effects.4 Recent innovations emphasize continuous peripheral nerve blocks with scheduled (acetaminophen and tramadol) and prn (oxycodone) analgesics; no intravenous opioids are administered. In accordance with strict criteria, 90% of patients undergoing minimally invasive hip (or knee) replacement using a comprehensive, preemptive, multimodal analgesic regimen emphasizing peripheral nerve block achieved readiness for hospital discharge within 48 hours.2 When a similar regimen was applied to patients undergoing standard total joint replacement, significantly improved perioperative outcomes were reported, along with fewer adverse events, compared with patients receiving traditional intravenous opioids during the initial postoperative period. Improved perioperative outcomes include a shortened hospital length of stay and a significant reduction in postoperative urinary retention and ileus formation.19 Finally, because hospital costs appear to be directly related to the length of hospital stay, analgesic techniques associated with improved recovery and reduced complications may decrease total direct medical costs among these patients. The reduction in mean cost is primarily associated with lower hospital-based (Medicare Part A) costs, with the greatest overall cost difference reported among patients with significant comorbidities.17 These studies support the movement toward continuous peripheral technique as the optimal analgesic method following total knee and hip arthroplasty.
Protocols used at the Mayo Clinic and other centers with respect to preoperative, intraoperative (pain injection cocktail), and postoperative multimodal pain regimens are shown in Table 27-4. It is important to note that no formal comparison between clinical pathways has been performed. Likewise, critical elements within a given clinical pathway are not easily determined because of the large numbers of component and system adaptations that typically occur during implementation of a new practice model. Institutions proposing to initiate a recovery/rehabilitation pathway should consider the critical concepts of a multimodal approach: limit opioid administration and thereby reduce the frequency/severity of opioid-related side effects (through administration of nonopioid analgesics, performance of regional techniques and local infiltration, and use of other nonpharmacologic therapies such as ice application), and adapt them to the institution’s practice model. For example, the local anesthetic infusion concentration/infusion rate in patients with peripheral nerve catheters may be adjusted according to the amount of weight bearing desired.
Table 27-4
Mayo Clinic Total Joint Regional Anesthesia Clinical Pathway
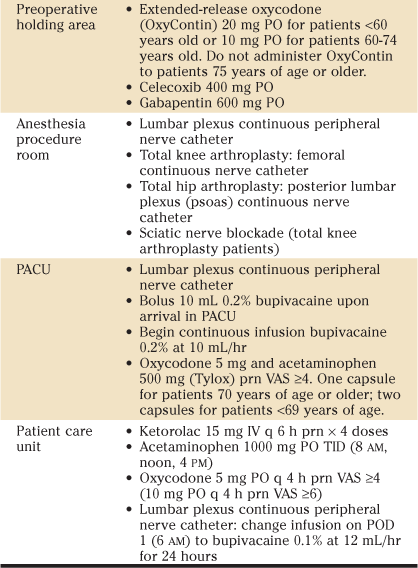
BID, Twice a day; IV, intravenous; PACU, postanesthesia care unit; PO, per os; POD, postoperative day; prn, as necessary; TID, three times a day; VAS, verbal analogue pain score.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








