Biological Responses to Particle Debris
Stuart Goodman and Ting Ma
Key Points
Introduction
Overview
Wear—loss of material from the surfaces of the bearing couple—inevitably occurs with use of total joint replacements, leading to the production of particulate debris. According to McKellop, hundreds of thousands to millions of particles are produced from a metal-on-conventional-polyethylene total hip replacement with each step.1 The body normally distributes this debris locally and regionally via phagocytosis of particles by macrophages and other cells, leading to activation of cells to produce proinflammatory mediators. Despite the ongoing generation of small amounts of particulate debris, a state of homeostasis normally exists in the joint and surrounding tissues, and the minor localized biological reaction to wear debris has minimal consequences.2 However, when the host reaction to wear debris and associated by-products is excessive and persistent (state of decompensation), chronic inflammation can lead to periprosthetic bone loss by biological processes that increase bone degradation and inhibit bone formation. This pathologic process is called particle-associated periprosthetic osteolysis or, among joint replacement surgeons and researchers, simply osteolysis. Although we normally think of osteolysis as a radiologic phenomenon (loss of bone on a radiograph of a joint replacement on follow-up), osteolysis is a complex pathophysiologic process with potentially major adverse clinical implications. This chapter will review the basic science underlying the biological reactions to wear debris from polymeric, metallic, and ceramic implants.
Basic Science
The Bone-Implant Interface: Histology, Cell, and Molecular Biology
Regardless of whether a hip prosthesis is cemented or cementless, or contains a bioactive surface coating, long-term implant stability within bone is a prerequisite for optimal pain-free function. During the “surgical trauma” of prosthesis implantation, an inflammatory response is initiated in which proinflammatory mediators are released locally. This begins a cascade of events resulting in the resorption of dead bone and marrow contents, and the accretion of new bone immediately surrounding the implant.3 Over the ensuing weeks to months, the prosthesis becomes surrounded by a network of bony trabeculae, which undergo remodeling according to local biological influences and mechanical loads. Thus, the bone-implant interface is a dynamic structure that matures and remodels over time, long after the prosthesis is implanted. Long-term prosthesis stabilization is possible whether the method of fixation is cemented or cementless.4–6 However, if the cement mantle fractures and degrades, or if excessive amounts of wear debris are produced from articulating and nonarticulating interfaces, an aggressive chronic inflammatory reaction can develop in response to foreign bodies, which may result in periprosthetic osteolysis7–13 (Fig. 11-1).
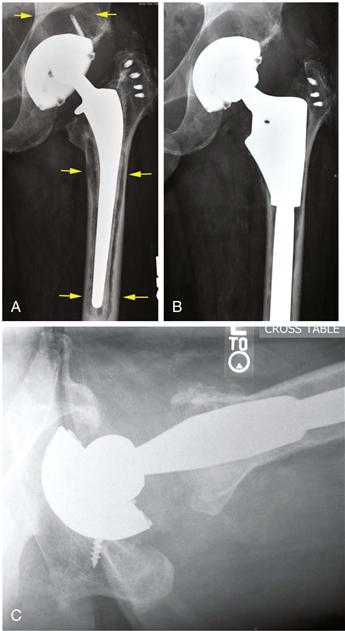
Figure 11-1 Total hip replacement with polyethylene wear, acetabular osteolysis, and loosening and osteolysis around the cemented femoral component. A, Preoperative radiograph. The upper arrows point to the acetabular osteolysis. Note the eccentrically positioned femoral head indicating wear of the plastic. The cemented femoral component is loose, and the prosthesis has subsided within the bone. The cement mantle has fragmented. Cement debris has led to scalloping of the surrounding bone (lower arrows). Postoperative anteroposterior (B) and lateral (C) radiographs of the revised hip replacement. The osteolytic areas in the acetabulum were bone grafted, and the acetabular plastic liner and femoral component were changed.
Examination of periprosthetic tissues retrieved at surgery has allowed identification of some of the key histologic characteristics and biological mediators of bone destruction and remodeling. If the prosthesis is well fixed to bone, there little tissue is often found at the interface. This is true whether the prosthesis is cemented or cementless.6 The fibrohistiocytic tissues surrounding loose implants (with or without radiographic osteolysis) usually contain debris from various materials implanted in the operative site9,14,15 (Fig. 11-2). These particles vary in size from the submicron (exceeding the visual capabilities of the light microscope) to larger shards of particles often several hundreds of microns in length. As we will see later, particles in the phagocytosable range, up to about 10 microns in size, and especially those around 1 micron in size or smaller, appear to be most stimulatory to cells.
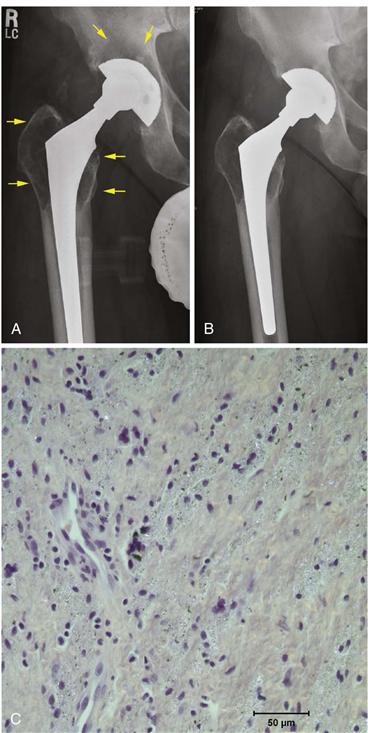
Figure 11-2 Total hip replacement with polyethylene wear and osteolysis. A, This metal-on-polyethylene cementless total hip replacement demonstrates eccentricity of the head within the polyethylene liner, indicating substantial wear of the plastic. Extensive osteolysis is seen around the metal socket and in the proximal femur (arrows). B, Postoperative radiograph after polyethylene liner and femoral head exchange. The osteolytic lesions were débrided and then filled with bone graft. The acetabular shell and the femoral component were well fixed and in satisfactory position. C, Photomicrograph of histologic section from tissue retrieved from granulomatous synovial capsular tissues demonstrates sheets of macrophages in a fibrovascular stroma. Abundant granular white specks of polyethylene and black metal particles are seen (hematoxylin and eosin stain, polarized light, ×40 magnification).
Willert and associates first described the biology of prosthetic stabilization and recognized that continuous production of wear particles involved many cell types from hematopoietic and mesenchymal cell lineages. Normally, a state of prosthesis-tissue homeostasis exists, and the particle load that is generated is dealt with by normal clearance mechanisms without adverse consequences.2 However, if these mechanisms are overwhelmed (state of decompensation) as the result of excessive production of debris or other causes, osteolysis due to cellular activation and bone resorption will ensue. It is interesting to note that Willert described the pathophysiology of prosthetic loosening long before others postulated more widespread distribution of particles into an “effective joint space,” or that increased hydraulic pressure with loading of the limb resulted in fluid flow around the prosthesis, distributing inflammatory cells and factors more extensively.13,16-18 Charnley first believed that areas of osteolysis around hip replacements were the result of low-grade infection.5
Cemented metal-on-polyethylene hip implants that are still well functioning may already demonstrate histologic findings consistent with excessive wear particle production.9 In autopsy specimens, the density of macrophages and foreign body giant cells in periprosthetic tissues was found to correlate with time after prosthetic implantation, membrane thickness, and polyethylene particle density. Wear particles in the joint gain access to the edges of the prosthesis interface, and then migrate in centripetal fashion through the fibrous interface and the cancellous bone.13,19 Examination of tissues harvested from cemented implants has revealed that the most critical factor associated with radiologic osteolysis was the concentration of polyethylene (PE) particles in the membranous tissue interface.20 In cementless implants, fibrous tissue present at the prosthetic interface functions as a conduit for migration of particles from the joint articulation into immediate periprosthetic tissues.21
Goldring and colleagues were among the first to apply the techniques of cell biology to tissues retrieved from revised implants.14,15 Using histologic, cell, and organ culture methods, they showed that tissue around loose cemented prostheses contained a pseudosynovial layer adjacent to the cement. Particles of polymethylmethacrylate (PMMA) bone cement within the membrane were found to be surrounded and engulfed by mononucleated and multinucleated macrophages and foreign body giant cells in a fibrovascular stroma. Lymphocytes were seen more rarely. Culture and analysis of the membrane showed that it had the capacity to produce large amounts of prostaglandin (PG)E2 and collagenase and enhanced bone-resorbing activity. Since these seminal studies were conducted, numerous investigators have shown that osteolytic membranes from failed prostheses express numerous degradative enzymes, cytokines, chemokines, nitrogen oxide and superoxide metabolites, and other proinflammatory and anti-inflammatory molecules22–31 (Fig. 11-3).
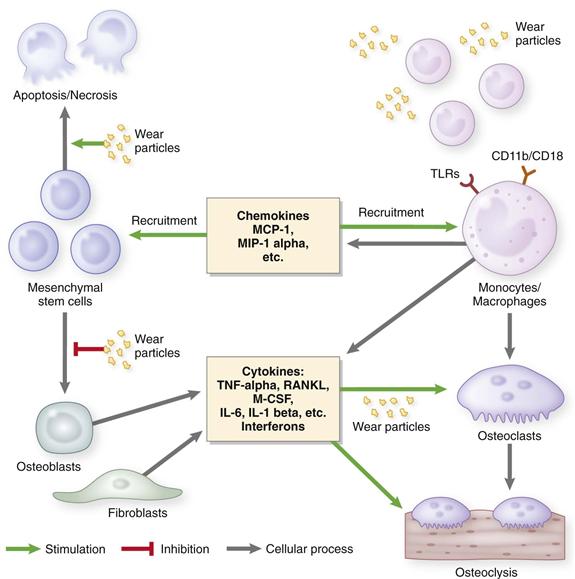
Figure 11-3 Diagram of the biological reactions associated with wear particles.
In rare cases, an aggressive granulomatous reaction develops, with localized, progressively expanding areas of bone lysis containing activated macrophages, fibroblasts, and other cells. It is postulated that these cases may be due to an uncoupling of events that normally lead to monocyte-macrophage clearance of wear particles via the nonspecific foreign body reaction and fibroblast-mediated formation and remodeling of the extracellular tissue matrix.32–34 However, the periprosthetic tissues are heterogeneous; biopsies from different areas may yield different histologic and cytokine profiles.35 It is interesting to note that the bony interface surrounding loose hip and knee prostheses presents histologic evidence of both bone destruction and active bone formation, which implies that even during osteolysis, an active reparative process is ongoing.36 Thus, heightened alkaline phosphatase activity, a marker of bone formation, has been noted at the bony surface surrounding areas of osteolysis.36
NFκB is a transcription factor that regulates numerous proinflammatory and anti- inflammatory pathways. Tumor necrosis factor-alpha (TNF-α) and interleukin-1β are two proinflammatory cytokines that play an important role in particle-associated periprosthetic osteolysis; they are under the regulatory control of NFκB. RANK (receptor activator of NFκB), a membrane-bound receptor on the surface of osteoclasts, activates the transcription factor NFκB. Receptor activator of nuclear factor kappa-B ligand (RANKL), a member of the TNF superfamily, is a receptor ligand that is released from osteoblasts, stromal cells, and other activated cells in the inflammatory process. RANKL interacts with RANK, which, together with macrophage colony-stimulating factor (M-CSF), is essential for the differentiation and maturation of tissue monocytes/macrophages into bone-resorbing osteoclasts. RANKL is inhibited by the soluble decoy protein receptor antagonist osteoprotegerin (OPG). Studies have shown increased expression of RANKL and M-CSF and decreased OPG in the synovial fluid and periprosthetic tissues of revised prostheses exhibiting radiographic osteolysis.37–44
The host reaction to metal-on-polyethylene implants is generally thought to be both a nonspecific foreign body reaction and a chronic inflammatory reaction. Although attempts have been made to demonstrate an important role for T and B lymphocytes, it would appear that lymphocytes play an immunomodulatory rather than a primary role. Nevertheless, evidence of antigen presentation to lymphocytes has been reported in metal-on-polyethylene joint replacements.45,46 These biological pathways should be distinguished from the T cell–mediated, antigen-associated type IV allergic reactions seen in cases of metal-on-metal implants, which are discussed in Chapter 12.47–52
In Vivo Animal Models and In Vitro Studies
In Vivo Studies
Numerous in vivo animal models and in vitro studies have attempted to simulate the biological processes associated with particle-induced osteolysis and to identify the critical variables and mechanisms underlying particulate disease. Animal models have included various small and large species, with and without weight-bearing implants, particles with different material properties and characteristics, and harvest periods that have varied from hours to many months. Many of these in vivo models have been summarized in recent review articles22,31,53-56 (Table 11-1). It is often difficult to make definitive statements about particle-induced osteolysis on the basis of in vivo animal models because of the different variables used in each model and the relatively short duration of animal studies (weeks and months) compared with the period required for development of clinical and radiologic osteolysis in humans (years). However, experiments performed in animal models have provided some useful concepts that can be generalized to periprosthetic reactions in humans.
Table 11-1
Animal Models Currently Used to Study Wear Debris–Induced Inflammation
| Species | Type of Model | Key References |
| Mouse | Calvarial | 148, 188, 189 |
| Mouse | Air pouch | 127, 128, 190 |
| Mouse | Intramedullary implant with particles—femur | 119–121 |
| Rat | Intra-articular or intramedullary—femur | 116, 118, 166, 191 |
| Rabbit | Intramedullary—femur | 192–194 |
| Rabbit | Harvest chamber—tibia | 62, 195, 196 |
| Dog | Pistoning implant – femoral condyle | 107 |
| Dog | Intramedullary–hip replacement | 175, 197, 198 |
| Sheep | Intra-articular injection–hip replacement | 199 |
The biological pathways involved in foreign body and chronic inflammatory reactions are complex, redundant, and difficult to inhibit completely. As mentioned previously, numerous cellular constituents and inflammatory factors participate in this reaction. Furthermore, various characteristics, including the material, number, size, shape, surface area, topography, and surface chemistry of particulate debris, have been shown to influence the cellular and inflammatory profile.25,57-62 In general, bulk materials produce a more benign interface compared with the same volume of material in particulate form. Smaller particles that are phagocytosable, especially those around 1 micron or smaller, appear to be the most reactive. However, very small particles, less than about 0.3 microns, do not appear to activate cells as they undergo pinocytosis rather than phagocytosis. Smaller particles may agglomerate together and therefore become much larger than the individual particles. This is especially true for polymers such as polyethylene. Irregularly shaped particles are more activating than rounder particles. In general, excluding the type IV cell-mediated immune reactions that are sometimes seen with metals, particulate materials such as alumina ceramics evoke less of a foreign body and chronic inflammatory reaction compared with metals (e.g., titanium alloy, cobalt chrome alloy, tantalum) at the same dose.63
Particulate polymers such as polyethylene and polymethylmethacrylate evoke the most exuberant reactions. In one study in which PE particles incited an inflammatory response, the same concentration of cobalt chrome alloy particles caused cell necrosis.62 These experiments are controversial though because of difficulties involved in obtaining and exposing cells to particles of similar doses, shapes, surface areas, surface chemistries, and so forth. For example, highly cross-linked PE particles have been found to be more bioreactive (i.e., they produce more inflammatory cytokines) than similar particles of conventional polyethylene in vitro.58,60 However, in vivo wear of highly cross-linked polyethylene is far less than that of conventional polyethylene, thereby presenting a much lower particle load to the body. It is clear that there is a need for standardized in vitro and in vivo models using standardized particles with known characteristics to more clearly delineate the biological effects of particles.
In Vitro Studies
In vitro studies performed over the past 20 years have clearly shown that wear particles can activate cells, resulting in the production of proinflammatory cytokines, chemokines, prostanoids, nitrogen oxide and superoxide metabolites, degradative enzymes, and other molecules. Indeed, the production of proinflammatory and anti-inflammatory factors is determined by the local presence of sufficient numbers of wear particles. In other words, there is a dose-response relationship between the amount of debris and the degree of cell activation. The type and magnitude of the reaction are also governed by the particle material, size, and shape, as well as by topography, surface area, surface chemistry and energy, contamination with other ligands, and other factors.* Indeed, particles do not necessarily have to be phagocytosed to activate cells; the particle-protein complex is able to activate cells by interacting with the cell surface integrins.71
In past experiments performed in vitro to study particle-induced cell activation, much emphasis was placed on traditional proinflammatory cytokines, such as TNF-α, interleukin (IL)-1 beta (IL-1β), and IL-6.78–80 However, the list of proinflammatory and anti-inflammatory mediators released from particle-exposed cells is almost endless. Furthermore, although macrophages have generally been used for in vitro cell stimulation studies, mediator release has been documented for other cell types, including fibroblasts,31,56,81-87 osteoblasts,85,88-94 and other cells. Indeed, some particles appear to be more toxic for macrophages than fibroblasts.95
Osteoblasts are derived from mesenchymal stem cells (MSCs), which become osteoprogenitors after further cellular differentiation. Recent research has shown that MSCs and osteoprogenitors are adversely affected by orthopedic materials in particulate form.25,96 These adverse effects have been shown for particles of titanium, cobalt chrome, polymethylmethacrylate and polyethylene.97–102 In general, particles in sufficient doses suppress MSC proliferation, differentiation, and maturation. This is reflected in decreased total DNA, decreased expression of osteoblastic markers such as osteocalcin and osteoblast-specific transcription factors, and decreased calcified matrix as seen in the von Kossa stain.
Pressure.
Although it is beyond the scope of this chapter to review the effects of pressure on cells in general, in the context of implant fixation, pressure can also cause alterations in local bone remodeling, including new bone formation and osteolysis.103 This fact has been known for some time: clinical cases in which implants have subsided during loading are often associated with pressure-induced widening of the medullary canal, thinning of the cortices, and the presence of a bony pedestal distal to the implant. During each step, hip and knee implants are loaded in a way that is dependent on the magnitude and direction of the loads, the anatomic shape, the mechanical characteristics, and the material properties of the bone and prosthesis.
Normally, a thin layer of synovial fluid bathes the natural or replaced joint articulation; this fluid is pumped into contiguous accessible areas of the joint, also known as the effective joint space, during loading of the limb, much like a hydraulic piston. Increased fluid production within the joint (synovitis) will cause increased intra-articular pressure during loading; this pressure will be transmitted to the joint space and contiguous areas. An increased number of wear particles may overwhelm the local homeostatic mechanisms, resulting in transport of fluid laden with wear particles, cellular infiltrates, proinflammatory factors, and other molecules into the effective joint space.2,13 This may lead to osteolysis remote from the source of particle generation, as is seen in cases of polyethylene wear with widespread osteolysis around the femoral stem.
In vivo animal studies have confirmed that pressure alone can lead to bone remodeling, mostly in an adverse fashion.16-18,104,105 Indeed particles and cyclical loading, pressure, and mechanical strain have been shown to be synergistic.106–108 Although these animal models are somewhat different from the clinical situation, they do illustrate the point that pressure can induce adverse bone remodeling in the absence of wear particles.
Particles, Endotoxin, and Bacterial By-products
When particles are generated, they are immediately coated with specific serum proteins.109,110 Bacteria and their by-products may also attach to particles. Many of the earlier in vitro and in vivo studies probably were performed with particles unknowingly contaminated with bacterial by-products. This is a serious problem because endotoxin and other bacterial antigens attached to orthopedic particles are powerful stimuli for cell activation and proinflammatory cytokine release.75,111-114 Studies have also shown that retrieved wear particles that have been processed to rid particles of endotoxin have blunted stimulatory effects on macrophages. When endotoxin-coated particles were introduced to the same cells, the cells became activated as evidenced by heightened proinflammatory cytokine release. One may criticize these studies on the grounds that strong acids and bases, substances that surely would alter the surface chemistry and energy of the particles, were used to rid the particle surface of endotoxin. As a result, particle-protein surface interaction with cells would be changed, possibly resulting in attenuated cellular activation. Nevertheless, the issue of implant contamination by bacterial by-products has gained increasing interest because of recent reports that lipopolysaccharide (LPS) has been detected on failed retrieved implants that were revised for supposed aseptic loosening.115 Whether remote bacterial contamination induced prosthetic loosening (despite the absence of overt bacterial infection at retrieval) or loosening preceded bacterial colonization of the prosthesis is currently unknown.
Recent Developments in Particle Disease: New Models and New Concepts
Clinically, ongoing production of excessive wear debris may be asymptomatic or may result in chronic synovitis with pain, swelling, and compromised joint function, periprosthetic osteolysis with pathologic fracture, or other local symptoms. Although newer alternative bearing surfaces will decrease the particle load delivered to local tissues, presently no successful nonsurgical pharmacologic methods are available to treat the adverse effects of wear particles. This fact has spawned new research into the pathogenesis of particle disease in the hope that early nonsurgical intervention may mitigate the clinical symptoms and signs and may delay revision surgery. Several new in vitro and in vivo models have further delineated the biological pathways involved in wear particle disease. Furthermore, new preclinical pharmacologic treatments for osteolysis have been proposed and tested in the laboratory.
Models of Continuous Delivery of Particles In Vivo
In humans, wear particles are produced continuously with use of the joint. Therefore, local tissues are exposed to wear debris over prolonged periods of time, despite the body’s attempts to rid itself of the particles. Most animal models have incorporated a single application of particles or multiple periodic injections of particles to an anatomic site, which is unlike the clinical situation. Kim and colleagues used a diffusion pump to deliver PE particles to the knee joint containing an intramedullary femoral Kirschner wire in rats, simulating continuous particle exposure.116–118 This novel model showed that continuous high-density PE particle infusion was associated with formation of an inflammatory periprosthetic membrane lined with osteoclasts, increased expression of TNF-α mRNA, and radiographic osteolysis around the rod.
Our group has optimized and validated a similar model using bench-top experiments, a murine femoral intramedullary infusion explant model, and in vivo murine infusion experiments.119–122 In the first experiment, we suspended two types of particles in mouse serum: polystyrene particles (dyed blue) and particles of ultra-high-molecular-weight polyethylene (UHMWPE) that had been retrieved from clinical cases. This suspension was then loaded into Alzet miniosmotic pumps (Durect Corporation, Cupertino, Calif) attached to hollow titanium rods via vinyl tubing.120 The number of particles delivered to a collection vessel was evaluated over 2- and 4-week time periods. Infusion of UHMWPE particles at clinically relevant dose levels yielded significantly more bone loss compared with controls, in which only mouse serum was infused. Finally, we infused clinically derived UHMWPE particles into the intramedullary space of the mouse femur for 4 weeks using a subcutaneous osmotic pump122 (Fig. 11-4). Infusion of UHMWPE for 4 weeks was associated with reduced bone volume and altered alkaline phosphatase expression. Continuous infusion of particles using the murine femoral implant model appeared to simulate the human clinical scenario of wear particle generation and delivery. This model has proved useful in studying the biological processes associated with wear debris.
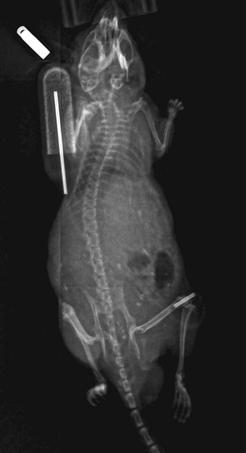
Figure 11-4 This radiograph of a mouse demonstrates a hollow intramedullary rod in the distal right femur to which is attached radiolucent tubing connected to an osmotic pump containing a solution of polyethylene particles (upper left of figure). The particles are driven from the pump into the tubing through the hollow rod into the distal femur.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







