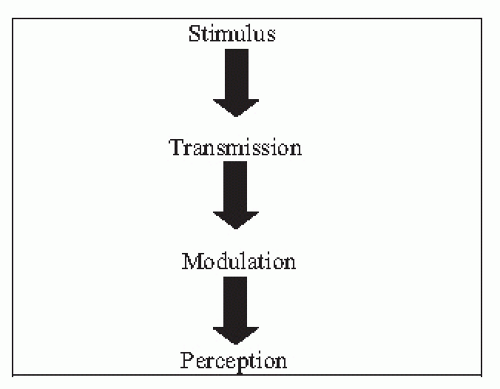
over the last 25 years. Particularly in the acute pain setting, these mechanisms are now understood and medical interventions that directly interrupt pain processing pathways are available.
TABLE 8.1 Systemic Effects of Acute Pain and the Postinjury Stress Response | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
TABLE 8.2 The Mechanisms of Chemical Nociceptor Transduction at the Stimulus Attack Point | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||
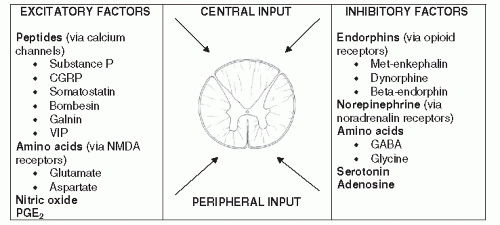
than the net excitatory signal, then the gate remains closed. If the cumulative excitatory signal is stronger than the net inhibitory signal, then the gate is opened and the signal is transmitted on (48,50,81,82,83,84 and 85).
noxious stimulus creates a cycle of inflammation and further sensitization. If noxious stimuli or peripheral sensitization are maintained, then central sensitization can be sustained indefinitely. In contrast, when the peripheral signal is removed, there is evidence that central sensitization resolves within minutes (44,47,98).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree