I. Scapular Fractures—Fractures of the body of the scapula in children occur infrequently, result from high-energy trauma, and are often associated with concomitant injuries of the thorax and chest. Treatment is generally nonsurgical (except for open injuries) and consists of an arm sling and range of motion exercises of the shoulder to decrease stiffness.
II. Injuries of the Clavicle and the Sternoclavicular and Acromioclavicular Joints
A. Clavicle—The clavicle is the first bone to ossify in the human embryo and one of the last to fuse its epiphyses to its diaphysis. The medial epiphysis ossifies at 12 to 19 years of age and fuses at 22 to 25 years of age. The lateral epiphysis fuses to the diaphysis at 19 years of age, is thin and difficult to see on plain radiographs.
1. Mechanisms of injury—The clavicle is the most frequently fractured bone in children. Fractures are generally caused by a direct blow, a fall on the point of the shoulder or outstretched arm or during delivery.
2. Evaluation
• Physical examination—In the newborn, the fracture may present only as pseudoparalysis of the upper extremity. This must be distinguished from a brachial plexus palsy. The fracture may not be apparent on plain radiographs until callus appears 1 to 2 weeks after injury. Other findings may include those seen in toddlers and children including crepi-tation, swelling, point tenderness, decreased shoulder motion, and the head turning away from the fracture.
• Imaging—Anteroposterior (AP) and 30° cephalic tilt radiographs (serendipity view) can be obtained. If inconclusive, computed tomographic (CT) scan, tomograms or stress views with weights (for suspected lateral fractures) may be helpful.
3. Associated injuries—Associated injuries are uncommon and are primarily neurovascular. Venous distention, absent pulses or numbness should be evaluated. Concomitant obstetric brachial plexus palsies can occur in the newborn.
4. Treatment—The primary purpose of the clavicle is to connect the trunk to the shoulder girdle. Because of the excellent healing and remodeling potential in children, open surgical treatment of clavicle fractures is rarely indicated with the exception of open fractures, fractures with severe tenting of the skin that may become open and fractures associated with neurovascular compromise. Parents should be warned about a visible residual bump of healing callus at the fracture site that will likely persist over time. Most fractures require simple immobilization of the shoulder girdle. This can generally be accomplished with a Velpeau sling or shoulder immobilizer in children and adolescents. In neonates, the affected extremity should be bound to the thorax by safety pinning the arm of the onesie to the vest of the onsie or by fashioning a custom immobilizer out of stockinette.
5. Complications—Cosmetic bump.
6. Differential diagnoses
• Congenital pseudoarthrosis of the clavicle usually occurs on the right side except in newborns with situs inversus. There will be no history of trauma.
• Cleidocranial Dysostosis affects the clavicle and other bones of intramembranous ossification including the skull, mandible, and vertebrae.
B. Sternoclavicular Joint—The clavicle articulates medially with the sternum and the first rib. Injuries in this region in children are usually Salter-Harris Types I or II physeal fractures rather than true joint dislocations. Posterior displacement of the medial clavicle can cause impingement on the innominate artery and vein, vagus and phrenic nerves, trachea, esophagus and/or brachial plexus. Anterior displacement is more common.
1. Evaluation
• Physical examination—A lump or depression at the sternoclavicular joint may be evident with tenderness, possible hoarseness, dyspnea, dysphasia, diminished affected upper extremity pulses or venous engorgement of the extremity.
• Imaging—On Serendipity view, posteriorly displaced separation will appear more caudad and anterior displacement will appear more cephalad. Because the medial epiphysis may not ossify until 19 years of age, CT scan may be required to adequately visualize the bony anatomy.
2. Treatment—Anterior sternoclavicular injuries generally require only symptomatic treatment with sling and swathe due to excellent remodeling potential. Asymptomatic posteriorly displaced injuries are closed reduced by placing a blanket roll longitudinally between the shoulder blades and placing posteriorly directed pressure on the lateral clavicle or shoulder thereby distracting, while simultaneously grasping the medial clavicle percutaneously with a pointed reduction forceps or towel clip under a general anesthetic. Some advocate having a pediatric or vascular surgeon available in case the displacement is tamponading a vessel. Open reduction is reserved for symptomatic irreducible posteriorly displaced injuries and is rarely required.
C. Acromioclavicular Joint—Injuries of the acromioclavicular joint in children are usually fractures, not dislocations. The coracoclavicular and acromioclavicular ligaments remain attached to the thick periosteal sleeve.
1. Classification (Dameron and Rockwood) (Fig. 31-1)
• Type I—Mild sprain without periosteal disruption.
• Type II—Partial disruption of the dorsal periosteal tube with some distal clavicle instability.
• Type III—Large longitudinal dorsal split in the periosteum with gross instability.
• Type IV—Similar to Type III; the distal clavicle is displaced posteriorly and buttonholed through the trapezius.
• Type V—Complete dorsal periosteal split with superior subcutaneous displacement through the deltoid and trapezius.
• Type VI—Inferior dislocation of the distal clavicle beneath the coracoid.
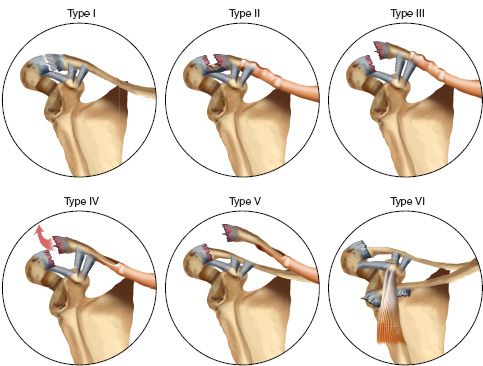
FIGURE 31-1 Acromioclavicular separation in children (Dameron and Rockwood classification).
2. Evaluation—Serendipity and AP radiographs or a CT scan can be obtained; stress views may be required due to bony overlap on the AP radiograph.
3. Treatment—Closed treatment with sling and swathe for Types I and II. Open reduction is indicated for Types III to VI in adolescents.
III. Injuries of the Humerus and Glenohumeral Joint
A. Glenohumeral Dislocations—Glenohumeral dislocations are uncommon in children but appear to be increasing with increasing sports participation. Treatment is similar to that for adults. The two primary predictors of recurrent dislocation are (a) number of prior dislocations and (b) age at first dislocation. Recurrence in children is very common.
B. Proximal Humerus Fractures—Fractures of the proximal humeral physis occur most commonly in adolescents secondary to high-energy sports participation and a weak perichondrial ring; in newborns, this injury most often results from a complicated delivery or child abuse. The distal fragment normally displaces anteriorly and laterally because of the strong posteromedial periosteum; the proximal fragment flexes, abducts, and externally rotates. Salter I and II fractures and fractures of the proximal humerus metaphysis occur most commonly between 5 and 12 years of age. Pathologic fractures may occur secondary to unicameral bone cysts of the proximal humeral metaphysis.
1. Mechanism of injury—Mechanisms of injury to the proximal humerus include birth trauma (usually Salter I) and falls on an outstretched arm (usually Salter I or II or metaphyseal).
2. Classification
• Neer and Horwitz classification—based on displacement.
(a) Grade I—displaced less than 5 mm
(b) Grade II—displaced at least one-third of the shaft width
(c) Grade III—displaced at least two-thirds of the shaft width
(d) Grade IV—displaced more than two-thirds of the shaft width
• Pathologic fractures—Primarily occur through simple bone cysts of the proximal metaphysis. Active cysts are within 1 cm of the physis and latent cysts are greater than 1 cm from the physis.
3. Evaluation
• Physical examination—Pseudoparalysis, tenderness, swelling, and pain may be present.
• Imaging—Fractures with or without displacement are frequently seen on plain radiographs. Because the proximal epiphysis does not ossify until 6 months of age, newborn physeal separations may appear as an abnormal relationship between the scapula and humerus on plain radiographs. Ultrasonography is beneficial in these patients. Pathologic fractures through bone cysts can be confirmed by MRI.
4. Treatment—Most proximal humerus fractures can be treated by closed means. Eighty percent of the longitudinal growth of the humerus comes from the proximal physis resulting in significant remodeling potential. In addition, the large range of motion of the shoulder joint allows for minimal loss of function despite nonanatomic reduction. Acceptable limits of reduction in adolescent proximal humeral fractures include angulation of 35° and bayonet apposition. If the displacement exceeds these recommendations, closed reduction should be attempted. The shoulder should be immobilized in a shoulder immobilizer if stable, in a spica cast in the “salute position,” or with percutaneous pinning. Open reduction may be needed in severely displaced fractures and in open fractures. Common impediments to closed reduction include the biceps tendon and periosteum. If no reduction is required, the extremity should be immobilized in a sling and swathe, or stockinette in newborns and infants, as previously described for obstetric clavicle fractures. Minimally displaced pathologic fractures through cysts are initially treated symptomatically with a sling. Latent and active cysts are treated with serial aspiration and steroid injections or bone marrow injection until they resolve. Curettage and bone grafting results in more reliable healing but may lead to growth arrest in active cysts.
5. Complications—Complications include growth arrest, diminished shoulder motion, malunion, recurrence of a cyst, and refracture.
C. Humeral Shaft Fractures—Fractures of the humeral shaft are uncommon. Fractures involving the proximal and distal metaphyses are more common.
1. Incidence—Humeral shaft fractures occur more commonly in infants and toddlers and in adolescents older than 12 years.
2. Mechanism of injury—Pediatric humeral shaft fractures may result from birth trauma, torsional forces (child abuse), direct trauma, falls, or from throwing activities.
3. Evaluation—In newborns or infants, irritability and pseudoparalysis may be the only indicators of a recent fracture. The only physical finding may be a palpable lump that manifests 7 to 10 days after the initial injury. In a child or toddler, there may be pain, swelling, and an inability to use the extremity. Although most of these fractures result from accidental trauma, other evidence of child abuse should be sought if there is a suspicion of nonaccidental trauma. Fractures of the humeral shaft can also occur through areas of compromised bone strength such as a cyst, nonossifying fibroma or other lesions. De novo spiral fractures of the humerus can occur in adolescent throwing athletes. The fracture is typically long, and the degree of displacement is small due to a thick periosteum.
• Infants and children—A shoulder immobilizer, sling and swathe, or hanging arm cast is usually sufficient. Skeletal traction is rarely indicated. All states mandate reporting of suspected child abuse by attending physicians, residents, physician assistants, and nurses.
• Adolescents—Nonsurgical treatment including functional bracing, hanging arm cast, or coaptation splinting should allow for acceptable healing in the vast majority of cases including fractures resulting from throwing. Open treatment including plating or intramedullary nailing is rarely indicated unless an acceptable closed reduction is not achievable or in multitrauma patients. Frequently, unacceptably angulated and displaced humeral shaft fractures will reduce to an acceptable position over the course of one week as the muscle spasm relaxes secondary to gravity and a sling or coaptation splint.
5. Complications
• Overgrowth—Mild overgrowth occurs in 80% of humeral shaft fractures in children but is rarely significant.
• Radial nerve injury—Radial nerve injuries may occur with fractures at the junction of the middle 1/3 and distal 1/3, but are infrequent. Radial nerve dysfunction that is present initially after injury should be observed for recovery on physical examination (likely a stretch injury). Nerve dysfunction that occurs only following attempted closed reduction may prompt consideration for surgical exploration of the radial nerve (possible entrapment). These guidelines remain controversial, however.
IV. Injuries of the Elbow Region (Figs. 31-2 Through 31-10)
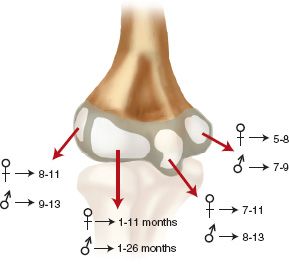
FIGURE 31-2 Age of appearance of the ossification centers around the elbow.
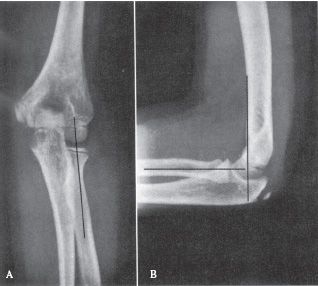
FIGURE 31-3 Radiographic lines for the evaluation of pediatric elbow injuries. A line drawn along the long axis of the proximal radius should bisect the capitellum on both the AP (A) and lateral (B) views. A line drawn along the anterior cortex of the distal humerus on the lateral view (B) (anterior humeral line) should bisect the capitellum. Disruption of these normal radiographic relationships is suggestive of injury. (Reprinted with permission from Brinker MR, Miller MD. Fundamentals of Orthopaedics. Philadelphia, PA: WB Saunders, 1999, with permission.)
A. Transphyseal Fractures (Distal Humeral Physeal Separation) (see Fig. 31-6)
1. Incidence—Transphyseal fractures generally occur in children 3 years old and younger.
2. Mechanism of injury—Transphyseal fractures occur as a result of a fall on an outstretched arm or from birth trauma but may occur secondary to child abuse in up to 50% of children under 2 years of age.
3. Classification (DeLee)
• Type A—A Salter I fracture occurring before ossification of the lateral condyle epiphysis, usually before age 1 year.
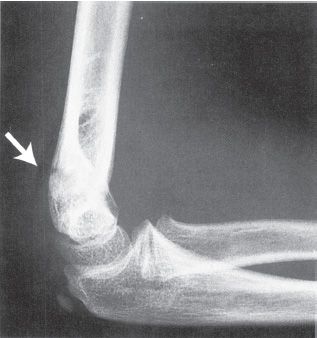
FIGURE 31-4 Posterior fat-pad sign (arrow) suggests the presence of an intra-articular effusion and fracture of the elbow. (Reprinted with permission from Brinker MR, Miller MD. Fundamentals of orthopaedics. Philadelphia, PA: WB Saunders, 1999, with permission.)
• Type B—A Salter I or II fracture with a small fleck of lateral metaphysis generally occurring between the ages of 7 months and 3 years.
• Type C—A fracture that includes a large metaphyseal fragment (Salter II) either medially or laterally, and generally occurring between ages 3 and 7 years.
4. Evaluation
• Physical examination—This injury should be suspected in any infant with a swollen elbow and irritability. This fracture has a larger surface area than the comparable supracondylar humerus fracture, and so rotation and angulation tend to be less. This injury must be differentiated from an elbow dislocation.
• Imaging—the proximal radius and ulna are in anatomic relationship with each other but tend to displace posteromedially with lend respect to the distal humerus. The key to distinguishing this injury from an elbow dislocation is maintenance of the radial head–capitellar relationship (see Fig. 31-5). In children younger than 3 years of age, the capitellum may not be ossified making the diagnosis difficult. An ultrasound, a MRI, or an arthrogram may be required.
5. Treatment—Closed reduction and percutaneous smooth K-wire fixation (see technique for supracondylar humerus fractures) diminishes the occurrence of cubitus varus that has been reported following closed reduction and cast immobilization, especially in patients younger than 2 years of age. The arm should be immobilized in a long arm cast postoperatively. Open reduction is rarely required. Healing normally occurs by 3 weeks.
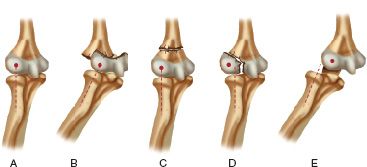
FIGURE 31-5 Radiocapitellar relationships. A. Normal elbow in which the long axis of the radius extends into the center of capitellum. B. Separation of the entire distal humeral physis (transphyseal fracture) in which the radiocapitellar relationship remains intact but the ossification center of the capitellum is posteromedial to the metaphysis of the distal humerus. C. Supracondylar fracture in which the radiocapitellar relationship is maintained. D. Fracture of the lateral condyle in which the capitellum is lateral to the long axis of the radius. E. Dislocation of the elbow in which the long axis of the radius is lateral to the capitellum.
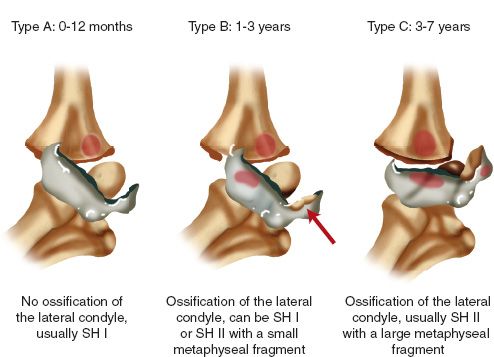
FIGURE 31-6 Transphyseal fractures of the distal humerus (DeLee classification): Type A, Type B, and Type C fractures.
6. Complications—Neurovascular injuries are less common than in supracondylar humerus fractures. Cubitus varus occurs following closed reduction and long arm cast immobilization and is diminished with percutaneous pin fixation. Avascular necrosis has also been reported.
B. Supracondylar Humerus Fractures (see Fig. 31-5)
1. Incidence—Supracondylar humerus fractures most commonly occur between 3 and 10 years of age. They are responsible for 50% to 70% of elbow fractures in children. They occur more frequently in boys than in girls. Injuries on the left side are more common than on the right.
2. Mechanisms of injury
• Extension injuries—Represent approximately 95% of supracondylar fractures. They are caused by a fall on an outstretched arm with the elbow in hyperextension. Posteromedial displacement occurs in approximately 75% while posterolateral displacement occurs in approximately 25%. The articulating surfaces of the distal humerus are connected to the shaft through a medial and lateral column. These two columns are separated by a thin area of bone formed anteriorly by the coronoid fossa and posteriorly by the olecranon fossa. This area is vulnerable to fracture when forced into hyperextension with the olecranon acting as a fulcrum.
• Flexion injuries—Flexion supracondylar fractures are caused by a fall on the posterior aspect of a flexed elbow.
3. Classification (Gartland)
• Type I—Nondisplaced or minimally displaced
• Type II—Displaced but with an intact posterior cortical hinge
• Type III—Completely displaced without cortical contact
4. Evaluation
• Physical examination—Patients with Type I or II fractures will complain of pain in the elbow especially with attempts at range of motion. Swelling is usually evident but may be minimal. In Type III fractures, there may be an obvious S-shaped deformity with significant amounts of swelling and ecchymosis. Puckering of the anterior skin generally indicates severe displacement with fracture penetration into the subcutaneous tissues. This may evolve into an open fracture. Detailed neurologic examination is required as there is a 10% to 15% incidence of neurologic injury. In addition, there is a 5% incidence of concomitant ipsilateral fracture, usually of the distal radius.
• Imaging—AP and lateral elbow views should be obtained. The lateral should be obtained in external rotation, as these fractures most frequently are unstable in internal rotation. On the lateral X-ray, the anterior humeral line (a line extending distally from the anterior humeral cortex) should intersect the middle third of the capitellum (see Fig. 31-3). Comparison views of the opposite elbow can also be obtained. Multidetector CT (MDCT) scanning can be obtained in the presence of a positive posterior fat pad sign (see Fig. 31-4), pain with range of motion, and a history of trauma if no fracture is readily identified. The scan can be obtained in a cast with comparable radiation exposure to a standard elbow series (minimal scatter with CT) when the arm is held above the head with the head tilted out of the field in the prone position. The rapidity of the MDCT scanning technique obviates the need for sedation even in the youngest child.
• Vascular—The brachial artery can be torn or, more commonly, set into spasm in and a Type III extension fracture. In a cold, pale, and pulseless extremity with a displaced supracondylar humerus fracture, initial management should include urgent fracture reduction in order to reconstitute the blood flow to the distal extremity. Preoperative arteriograms are contraindicated because they only delay treatment and do not alter the treatment plan. Capillary refill and pulse oximetry are unreliable in this situation. Doppler and manual palpation of the radial and ulnar arteries should be undertaken. If the ischemia does not resolve with closed reduction, open exploration is required. Brachial artery lacerations should be repaired or bypassed by a pediatric vascular surgeon. Arterial spasm can often be quelled by use of papaverine or 20% lidocaine directly applied to the area of spasm (not injected) under direct visualization.
• Neurologic—Documentation of a thorough motor and sensory examination of the involved extremity should be undertaken. This evaluation should include anterior interosseous nerve (AIN) function (ability to flex the distal interphalangeal joint of the index finger and the interphalangeal joint of the thumb; remember this is a motor branch so there is no sensory loss). Nerve injuries occur in 7% to 15% of these fractures. AIN palsy is the most commonly occurring nerve injury with extension-type supracondylar fractures. Radial nerve injury has been reported more commonly with posteromedially displaced fractures and median nerve injuries with posterolateral displacement. Ulnar nerve injuries can occur with flexion supracondylar fractures but more commonly occur iatrogenically as a result of medial pin placement.
6. Treatment—There are no standard guidelines for the amount of displacement and angulation acceptable for a supracondylar humerus fracture.
• Extension injuries
(a) Type I—These nondisplaced or minimally displaced fractures require long arm casting with the elbow in approximately 90° of flexion for 3 to 4 weeks.
(b) Type II—There is considerable debate over the proper treatment of these displaced fractures with an intact posterior cortical hinge. These have been traditionally treated with closed reduction and long arm casting. In order to avoid repeat displacement, studies have suggested that elbow flexion of 120° in the cast is necessary. Unfortunately, this may lead to ischemia of the distal forearm and hand because of swelling and may increase the incidence of compartment syndrome. In addition, malunion and cubitus varus are more common in the Type II fractures that are treated with closed reduction and casting. Many surgeons now prefer closed reduction and percutaneous pinning (CRPP) for all displaced supracondylar fractures. The advantage is that the elbow can then be immobilized at 90° of flexion or less which facilitates venous return. This author performs a closed reduction and assesses the vascularity of the distal extremity with the elbow flexed at 120°. If there is evidence of vascular embarrassment in this position, he proceeds to percutaneous pin fixation (see below).
(c) Type III—CRPP is the current standard of care. Many recent studies suggest that this does not need to be undertaken emergently in a patient with an uncompromised neurovascular status.
• CRPP technique—The patient is placed under a general anesthetic on a standard operating room table with lead covering the testicles/ovaries, breasts, and thyroid. Two arm boards are placed longitudinally along the operative side of the table. An image intensifier positioned vertically with the large flat collector portion pointing up from below is brought into the space between the two arm boards and left slightly lower than these. The child is then moved to the edge of the table so that the elbow is centered over the center aspect of the image intensifier collector. A closed reduction is then undertaken first applying longitudinal traction followed by correction of varus or valgus angulation, then rotation. The surgeon’s thumb is then placed over the olecranon, and the elbow is flexed and the forearm pronated. Image intensification views are then obtained in the lateral position (externally rotated) and oblique views are obtained to assess the medial and lateral column reduction. Once reduction is obtained, the elbow is assessed for stability in approximately 120° of flexion. If the hand blanches or the pulses are absent, the surgeon proceeds to percutaneous pin fixation. (If acceptable reduction cannot be achieved, the surgeon proceeds to open reduction.) With the elbow in extension, the author then preps and drapes the entire affected upper extremity including the axilla and the deltoid region using split sheets. The image intensifier is draped in as part of the table. The surgeon then repeats closed reduction. Once anatomic reduction is achieved, the surgeon pronates the forearm and tapes the arm to the distal forearm in maximal flexion with sterile Coban wrap. A smooth K-wire (usually 0.062) appropriate for the size of the elbow is inserted under biplanar fluoroscopy from the lateral epicondyle percutaneously across the fracture site and anchored just through the contralateral proximal humeral cortex. A second comparably sized smooth K-wire is then placed parallel to the first approximately 1 cm apart from it in the same manner. The Coban wrap is then released and the elbow is allowed to extend, and true AP and lateral image intensification views are obtained. Assuming that these are satisfactory, the surgeon then externally rotates the elbow and, under real-time fluoroscopy, flexes and extends the elbow to assess for instability. If any motion is present, the two lateral pins are supplemented with a medial pin placed with the elbow in extension by milking the ulnar nerve posteriorly into the ulnar groove and making a small incision over the medial epicondyle and spreading down to bone with a snap. The stability is then reassessed on the lateral view. The pins are then cut and bent external to the skin for incorporation under a cast or splint. A posterior splint can then be placed for one week or, alternatively, a long arm cast can be placed with the elbow in approximately 70° of flexion to facilitate venous return. This is possible because of the stability achieved with percutaneous pinning. If concerns over swelling exist, the cast can be either univalved down the volar aspect or bivalved. Three to four weeks of immobilization is required, and the pins can be removed in the office setting. No further immobilization is needed. Cross-pinning of the supracondylar fracture (one medial and one lateral) is the most biomechanically stable, followed by two parallel lateral pins, followed by two lateral pins crossing at or near the fracture site. A medial pin places the ulnar nerve at risk either during insertion or postoperatively by chronic tenting of the nerve over the wire with the elbow in flexion. The risk to the ulnar nerve during insertion can be diminished by inserting the pin under direct vision with a small incision or by placing the pin with the elbow in extension as described above.
• Open reduction—Indications for open reduction include open fractures, vascular compromise, or a fracture that cannot be adequately reduced closed. The surgical approach should be based on the surgeon’s opinion as to what is obstructing fracture reduction. The approach may be medial, lateral, combined, or anteriorly based. A posterior triceps splitting incision is contraindicated in extension supracondylar fractures.
• Traction—Indications for traction include an inability to reduce or to stabilize a fracture and severe swelling. The advantage is the ability to check vascular status. The disadvantages include difficulty in controlling the position of the child in bed, difficulty of obtaining AP X-ray imaging, difficulty achieving fracture reduction and cost of hospitalization. In general, a manual reduction under general anesthesia will still be required.
• Flexion injuries—Similar principles apply as for extension injuries except that flexion injuries are reduced and stabilized in extension given the greater likelihood of an intact anterior periosteal hinge. Because of the difficulties in achieving an adequate reduction, operative intervention is frequently indicated.
7. Complications
• Neurologic complications—Most neurologic complications involve a neurapraxia and recover spontaneously over weeks to months. If no improvement is seen 3 months after injury, exploration of the nerve can be considered. The AIN is a branch of the median nerve that provides motor innervation (no sensory) to the flexor pollicis longus (thumb IP joint flexion) and the flexor digitorum profundus to the index finger (index DIP joint flexion). AIN function should be tested pre-and postreduction as it is the most frequently injured nerve seen in extension-type supracondylar humerus fractures.
• Vascular complications
(a) If the extremity is pulseless, fracture reduction and stabilization are performed emergently. If the radial pulse is not restored by reduction and stabilization, an intraoperative arteriogram or direct exploration should be performed. If the radial pulse is absent but perfusion of the extremity appears normal (pink, pulseless hand) after reduction and stabilization, treatment options are controversial. These options include observation, immediate exploration, and delayed arteriogram.
(b) Compartment syndrome—Volkmann’s ischemic contracture was first described in 1881 as a consequence of the treatment of upper extremity fractures including supracondylar humerus fractures. This debilitating condition was later found to be the sequela of an unrecognized forearm compartment syndrome. Signs and symptoms of compartment syndrome of the forearm include pain out of proportion to the injury, pain with passive digital extension and lack of active digital extension. Classically the five P’s (pain, pallor, pulselessness, paresthesias, and paralysis) have been conveyed as diagnostic criteria; however, most of these occur late in the course of the disease after irreparable damage may have occurred. A high index of suspicion is required to make the diagnosis in a timely manner. Once considered, all circumferential dressings should be split and casts should be completely bivalved including under cast padding. Manometry of the muscle compartments and clinical examination determine the need for emergent fasciotomies of the forearm. In general, a volar compartment fasciotomy alone adequately decompresses both forearm compartments.
• Cubitus varus (gunstock deformity)—A cubitus varus deformity is the result of a malunion of a supracondylar fracture rather than an acquired growth deformity. While this deformity is primarily a cosmetic one, some recent evidence suggests it may present functional issues including the late development of a tardy ulnar nerve palsy. Nonetheless, supracondylar osteotomy for correction of a cubitus varus deformity will primarily result in improved cosmesis rather than improved function over the short term. Initial fracture treatment with CRPP decreases the incidence of cubitus varus.
• Stiffness—Almost all children eventually regain a normal range of motion following supracondylar humerus fracture. If significant stiffness persists beyond 3 weeks after cast removal, physical therapy is indicated. Occasional permanent stiffness may result from fibrosis of the muscles or elbow capsule or heterotopic ossification, especially in markedly displaced fractures
C. Medial Epicondyle Fractures
1. Incidence—Medial epicondyle fractures generally occur between 9 and 14 years of age. Approximately half of these fractures are associated with an elbow dislocation, which may reduce spontaneously. This may be the origin of the elbow stiffness so commonly seen following this injury.
2. Mechanism of injury—Mechanisms of injury for a medial epicondyle fracture include a direct blow to the medial aspect and the elbow, a valgus stress placed on the elbow and a sudden flexion-pronation muscle contraction resulting in avulsion. The ulnar collateral ligament of the elbow and the common flexor tendon of the forearm take origin from the medial epicondyle. The flexor-pronator mass serves as a deforming force on the fracture fragment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






