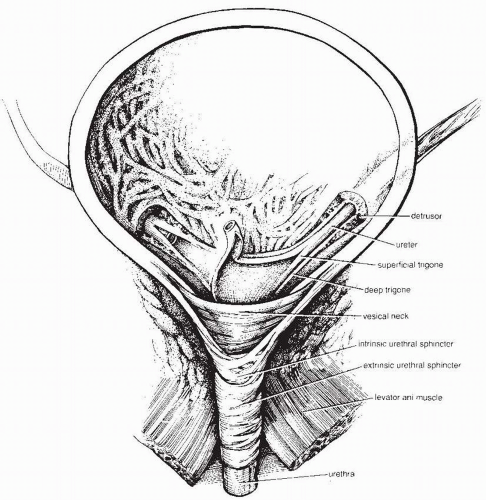
is under voluntary control. The striated muscular fibers in both man and woman are thought to have a significant proportion of slow-twitch fibers with the capacity for steady tonic compression of the urethra. In a woman, striated skeletal muscle fibers circle the upper two thirds of the urethra (9).
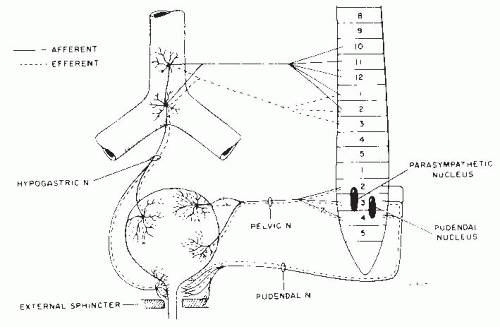
Beta-adrenergic receptors predominate in the superior portion (i.e., body) of the bladder. Stimulation of β-receptors causes smooth muscle relaxation. Alpha receptors have a higher density near the base of the bladder and prostatic urethra; stimulation of these receptors causes smooth muscle contractions and therefore increases the outlet resistance of the bladder and prostatic urethra (Fig. 51-3) (11, 12, 13).
These transmitters may work independently or help modulate the classic neurotransmitters. Nitric oxide and vasoactive intestinal polypeptide have smooth muscle relaxant effects. This helps explain the concept of atropine resistance. It has been found that a single neurotransmitter-blocking agent such as atropine fails to suppress 100% of the bladder or urethral activity (14,18). This explains why a combination of agents may be more effective than a higher dose of a single agent.
A replacement of normal muscle cell junctions by novel “protrusion junction cells” and ultraclose abutments, connecting cells into chains, which facilitates and increases spontaneous smooth muscle activity, has been found in incontinent elderly patients (30). There is often a combination of causes that result in an overactive bladder. Detrusor hyperactivity may coexist with impaired contractility (DHIC) of the bladder wall, resulting in both incontinence and retention. This combination was found to be one of the most common urodynamic findings in the elderly incontinent nursing home population (29). A combined pattern may also occur if there are cognitive/mobility issues and a person begins to void (incontinence) and then stops voiding.
the urodynamic terminology from International Continence Society Classification. Table 51-2 shows Wein’s classification. This classification is helpful at directing treatment because it is based on a clinical problem (incontinence or retention), which can then be applied to specific urodynamic findings. A urodynamic basic SCI data set has recently been developed, which should be helpful, collecting information in a standardized way regarding urodynamic bladder and sphincter function in those with SCI (40).
TABLE 51.1 Urodynamic Terminology—Highlights From International Continence Society Classification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 51.2 Urodynamic and Functional Classification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
been the most common urodynamic finding (72% to 100%) (49, 50, 51). Detrusor overactivity is thought to occur because of loss of the inhibitory input from the basal ganglia on the micturition reflexes; however, detrusor instability also has been associated with benign prostatic obstruction. Detrusor areflexia may result from bladder decompensation through a combination of bladder outlet obstruction and chronic use of anticholinergic and α-adrenergic medications (51).
activity with bladder filling. They believed that the patterns described by Blaivas et al. (62) represented variations of the single continence reflex.
creatinine clearance, and quantitative mercaptoacetyltriglycine (MAG) 3 renal scan and computerized tomography (CT). When ordering a test one must consider whether the information is needed about the function of the upper tracts or about the anatomy of the upper tracts. For example, a renal ultrasound is excellent to detect anatomical changes; however, it will not suggest any problems with renal function until hydronephrosis develops. Conversely, a renal scan detects stasis of the upper tracts before hydronephrosis develops, but is unlikely to detect a small kidney stone.
The inflammation is also likely to trigger uninhibited contractions and cause the bladder to be more overactive than usual. A recent prospective study found that 9.7% of SCI individuals who had asymptomatic bacteriuria developed a symptomatic UTI post testing. Nearly 40% of SCI individuals with sterile urine developed asymptomatic bacteriuria post testing (90).
or (b) the outlet (e.g., bladder neck, sphincter, or prostate) or retention caused by (a) the bladder or (b) the outlet. Management can be categorized as behavioral, pharmacological, surgical, or supportive. Table 51-3 shows various treatment options. Although each of these modalities is listed separately, it is important to note combinations within the same category (e.g., behavioral—timed voiding, fluid restriction, biofeedback) and separate categories (e.g., behavioral—timed voiding, pharmacologic—anticholinergics).
TABLE 51.3 Treatment Options for Voiding Disorders | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
medication is capsaicin, which is effective at suppressing uninhibited contractions for several months at a time (101). Unfortunately, capsaicin frequently causes discomfort or suprapubic pain, urgency, hematuria, and autonomic dysreflexia, which can last to up to 2 weeks postinstillation.
intestine, being careful to keep it attached to its mesentery, detubularizing it, and sewing it onto the bladder, which is first partially bivalved. Various bowel segments can be used and depend on the surgeon’s preference.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree