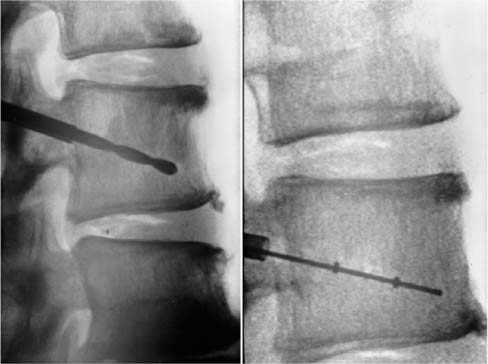
Chapter 34 The incidence of pathological vertebral fractures in the United States is approximately 700,000 per annum, 85% of which are associated with osteoporosis.1 The prevalence of osteoporosis of the lumbar spine in women aged 50 years or more is approximately 16% and, after correction for skeletal size, is similar for all ethnic groups.2 Osteoporotic fractures of the vertebrae have become increasingly recognized as a source of significant morbidity and mortality in an expanding elderly population.3 The clinical manifestations of osteoporotic vertebral collapse include pain, immobility, deformity (particularly in the sagittal plane), and rarely neurologic compromise. The negative emotional impact of vertebral fractures is more difficult to quantify but may be an even more important determinant of reduced quality of life.4 Traditional wisdom has declared that approximately two thirds of vertebral fractures are not symptomatic; however, this may have been underestimated. One study showed that 84% of patients with radiographically evident vertebral fracture reported associated back pain.1 The morbidity associated with osteoporotic fractures in general is considerable and it is estimated that 6.7% of women are rendered dependent in the basic activities of daily living because of osteoporotic fracture during their lifetimes.5 The association between hip fracture and decreased survival is now well established. Up to 20% of patients die in the year following hip fracture, representing a 5 to 20% reduction in expected survival.6 Recent studies indicate that vertebral fractures may also adversely affect survival. Clinically evident osteoporotic compression fractures were shown to be associated with a 15% age-adjusted increase in mortality not associated with clinically evident osteoporotic distal radial fractures.1 Kado et al7 prospectively studied 9575 women aged 65 years or more for a mean follow-up period of 8.3 years. Twenty percent of women were found to have one or more vertebral fractures. Compared to women who did not have fractures, these women had a 23% increase in age-adjusted mortality. The mortality risk was even greater (34%) for the 13.1% of women with severe fractures. Conventional treatment of osteoporotic vertebral collapse fractures has been almost exclusively non-operative. Bed rest, analgesia, nonsteroidal anti-inflammatory agents, and physical therapy with external bracing have been the mainstays of treatment. Operative reconstruction of the spinal column has traditionally been reserved for actual or impending neurological compromise and in the elderly population carries significant perioperative risk. Despite this conservative approach, or perhaps because of it, vertebral fractures in patients aged 65 years or older account for 150,000 hospital admissions in the United States each year. The monetary cost of treating osteoporotic fractures is enormous and is likely to increase with increased longevity of the population. The number of people in the United States aged 65 or more is expected to more than double from 32 million in 1990 to 69 million in 2050, and those aged 85 years or more are expected to increase fivefold from 3 million to 15 million.2 Direct medical expenses for osteoporotic fractures alone were estimated at $13.8 billion in 1995.8 Most of this (63%) was for treatment of hip fractures, although vertebral fractures accounted for only 5%. Any changes in treatment of vertebral fractures, particularly the use of new minimally invasive reconstruction techniques, are likely to have considerable cost implications. Osteoporotic vertebral fractures in addition to being a source of considerable distress to the individual patient may signify decrease survival, and this presents a challenging public health issue for the future. The purpose of this article is to review some of the newer, minimally invasive surgical techniques that have evolved for treatment of this condition. The terms vertebroplasty and kyphoplasty refer to percutaneous structural reinforcement of the vertebral body using polymethylmethacrylate acrylic cement (PMMA). This technique was initially pioneered in France by Galibert and colleagues9 over a decade ago for the treatment of vertebral hemangioma. Over the past 13 years the indications have been expanded to include metastatic disease of the spine and osteoporotic vertebral collapse. The procedure was first performed in the United States for osteoporotic vertebral fracture in 1995.10 Despite a small number of studies in the literature and the lack of prospective randomized trials, this procedure has gained increasing acceptance, particularly as a palliative measure in metastatic disease of the spine. One of the reasons for this has been the universal experience of prompt relief of pain in approximately 90% of patients treated using this method.9,11–14 Percutaneous vertebroplasty can be performed under general anesthesia, diazananalgesia (midazolam and fentanyl combined with local anesthesia), or local anesthesia.11 Prophylactic antibiotics are used in some centers but not universally.14 The percutaneous approach to the thoracic spine may be transpedicular or extrapedicular. The transpedicular approach is safe as long as the wall of the pedicle is not violated and in general is preferred if the diameter of the pedicle is large enough to accommodate the instruments. This approach minimizes the risks of cement tracking back along the path of the needle and, in the thoracic spine, minimizes the risk of pneumothorax. An extra-pedicular approach may be used if the pedicles are too narrow or cannot be cannulated for whatever reason. This can result in pneumothorax or leakage of cement along the needle track, which potentially could injure the nerve root exiting the foramen below. The transpedicular approach is essential in the lumbar spine to minimize the risk of cement tracking back around the exiting nerve roots. The patient is positioned prone on the operating table, which should incorporate a radiolucent spinal frame. High-quality biplanar imaging using fluoroscopy alone or a combination of fluoroscopy and computed tomography (CT) is essential. The pedicles are identified and an 11-gauge needle is introduced into the vertebral body using one of the approaches described. A spinal biopsy may be performed at this time using a trephine if required. Contrast medium is then injected into the vertebral body. This gives some indication of the local anatomy and venous drainage; however, it does not necessarily predict the casting of the cement, which has a different viscosity. The needle is advanced under fluoroscopic guidance to the anteroinferior border of the vertebral body to be treated. The cement, which must have been previously brought to room temperature, is then prepared. The cement is supplied as a liquid monomer and cement powder containing some polymer and must be mixed in sterile conditions. An agent to increase the radiodensity of the cement (such as tantalum powder) is added at the mixing stage. Once the cement has reached the consistency of a thin paste, it is loaded into 2 or 3 mL Luer-Lok syringes. The cement is allowed to solidify until it is just at the point where it can still be injected and is then injected under strict lateral fluoroscopy. The more viscous the cement, the less likely it is to migrate in an uncontrolled fashion. Injection is stopped immediately if cement encroaches on the posterior vertebral wall or is seen to migrate outside of the confines of the vertebral body. After vertebral filling, the stylet of the needle is reinserted and the needle slightly withdrawn to the cortical wall of the vertebra. Careful attention to the position of the needle during curing of the cement is essential to prevent fixation of the needle and rendering subsequent removal difficult. If 50% or less of the vertebral body has filled with cement, the procedure can be repeated on the opposite side. In general, a total of 2 to 6 mL of cement is injected at each level. The technique described above does not attempt to restore vertebral body height and thus correct the kyphotic deformity of vertebral collapse. This, at least in theory, may have pain implications from persisting deformity or abnormal biomechanical loading at adjacent levels. A procedure known as kyphoplasty has evolved to address this issue. This technique involves cannulation of the vertebral body using the trans- or extrapedicular approaches described above and insertion of an inflatable balloon tamp into the center of the collapsed vertebral body (Fig. 34–1). The balloon is then inflated using a device (Fig. 34–2) that delivers a controlled volume of radiopaque liquid (Figs. 34–3A through D). Inflation pressure is constantly monitored as inflation of the balloon is visualized on fluoroscopy. The object of this technique is to impact cancellous bone circumferentially around the tamp, exerting forces on the end plates above and below and thus reducing the deformity. Packing cancellous bone against an intact posterior vertebral wall should also decrease the likelihood of cement leakage into the spinal canal. The bone temp is then deflated and removed. The central defect is then filled with PMMA cement (Figs. 34–4, 34–5, 34–6). Filling of this defect should require less force than necessary for vertebroplasty, as the defect will offer no resistance to the insertion of cement. FIGURE 34–1 Balloons are inflated simultaneously under fluoroscopic guidance.
MINIMALLY INVASIVE SURGERY FOR
THE TREATMENT OF VERTEBRAL
COMPRESSION FRACTURES
SURGICAL TECHNIQUES
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree