Metastatic Disease Around the Hip
Joseph H. Schwab and Francis J. Hornicek
Key Points
• Surgery is often an integral part of the palliation of metastatic bone disease.
• The goal of surgery should be to allow immediate weight bearing.
Introduction
More than 1.5 million new cases of cancer will be diagnosed this year in the United States. It is estimated that 562,340 people will die from cancer in the United States in 2009.1 Lung, breast, and prostate cancers are the three most common forms of cancer; all three commonly spread to bone. Renal and thyroid carcinomas are also known to be osteophilic. Jaffe reported a 90% incidence of bone metastasis during autopsy of patients who succumbed to osteophilic tumor.2 Furthermore, the incidence of bone metastasis is expected to increase as the population ages. Although management of primary bone tumors is generally reserved for those specifically trained in orthopedic oncology, the sheer volume of bone metastasis mandates that all orthopedic surgeons should be aware of the principles of management. The purpose of this chapter is to introduce the reader to current concepts in the management of patients with metastasis about the hip joint. The management of bone metastasis is a multidisciplinary endeavor; therefore, nonsurgical management will also be discussed.
Epidemiology and Risk Factors
It is estimated that more than 14 million people worldwide are suffering from cancer, and that 70% to 90% of those with late-stage disease have significant pain.3 Bone metastasis contributes significantly to the prevalence of pain in this patient population. The axial and appendicular skeletons are the most common sites of metastatic disease. A report on more than 300 cases of bone metastasis stated that bones in and around the hip joint are involved in 66% of cases.4 Similarly, Galasko and associates found the pelvis to be involved in 66% of cases and the femur in more than half of cases of metastatic breast carcinoma.5
Paget was the first to popularize the concept that some tumors have a predilection for spreading to bone. At the time, competing views were put forth on how and where secondary cancers occurred. One popular theory of the time was that cells spread to other organs via the blood and became trapped in their parenchyma vis à vis an embolism. This theory was supported by Virchow, who believed that embolism was a critical means by which secondary cancers occurred. Paget disagreed with this view and mentioned the work of Langenbech, who thought that each embolic cell should be considered as a separate living entity. Further, he credited Fuchs, who noted that some organs were “predisposed” to secondary cancer. Paget stated, “When a plant goes to seed, its seeds are carried in all directions; but they can only live and grow if they fall on congenial soil.” He noted that breast and thyroid cancers have a predilection to spread to bone that cannot be explained by embolic theory alone.6
Subsequent studies have confirmed that some cancers spread to bone more frequently than others. Abrams and associates described their autopsy findings in 167 cases of breast cancer in which 73% of cases had bony involvement.7 The same report found that 32% of lung and 24% of renal cell patients had bony metastases at autopsy.7 Prostate cancer is well known to spread to the skeleton. When one is considering risk factors for metastatic disease to bone, the most likely tissues of origin include lung, breast, prostate, thyroid, and renal cell. Patients with a history of these malignancies are at higher risk for developing bony disease. Multiple myeloma should also be considered, because it is the most primary tumor in bone.
Pathophysiology
Although the location and speed with which tumors metastasize to other sites vary considerably, all tumor cells generally must clear five hurdles before they can grow in a different organ: cancer initiation, local invasion, circulation, infiltration, and colonization. Some cancers pass through these stages rapidly (such as non–small cell lung cancer, in which metastasis has often occurred by the time the primary tumor is detected).8 Others, such as breast and prostate, pass through these stages more slowly. Indeed distant spread may not be detected for years after the primary tumor has been detected and treated.9 In addition to the speed with which cancers spread, the location to which they spread is unique. Although breast commonly spreads to bone, it also commonly spreads to the liver and lungs. Prostate cancer, in contrast, generally spreads to the bones only late in the course of dissemination.10
Cancer initiation requires the cancer cells to grow in a heterogeneous way.11 For cancer cells to grow, they must be able to circumvent normal anatomic constraints that ordinarily would keep order. For instance, they must be able to break through the basement membrane and grow locally. This exposes cells to selective pressures that each cell must overcome. For instance, if a tumor is growing locally, it may outgrow its blood supply and then is exposed to a hypoxic environment. Some cells will not be able to grow in these conditions, and other cells adapt to the conditions by producing hypoxia-inducible factor. This triggers a series of events that allow the cell to grow and even thrive in this new hypoxic environment. This is an example of how cells might be “selected” to grow in an otherwise hostile environment. A key component of this concept is that cells within a colony are heterogeneous. Cells must display genomic instability for the heterogeneity to persist. So the progeny of the original aberrant cell may look very different genetically from that of its sister cells from the same original cell. If all cells in a colony were similar, then “selection” could not occur, because survival would be an all or none phenomenon.11
After cancer begins, cells must be able to break through their local basement membrane and grow into their local extracellular matrix. As mentioned, hypoxia is one environmental stress to which cancer cells may be exposed. Hypoxia stimulates, among other things, cancer cells to develop their own vascular supply. Some genes are known to be important in this stage of cancer development, including VEGF, MMP-9, and MMP-1.11 Another important component of this stage of cancer development is known as epithelial-to-mesenchymal transition.12,13 Epithelial cells generally respect the basement membrane as a boundary, and they have polarity that reflects their orientation to the membrane. Mesenchymal cells do not have the same polarity, nor do they respect the basement membrane as a barrier. One can see how this sort of transition would be favorable for the growth and spread of carcinoma.12,13
Once cancer cells have mastered their local environment, they must be able to enter the circulatory system and survive. At this point, it may seem only a matter of semantics whether metastasis has occurred or not. However, simply entering the circulation is no guarantee that metastasis will follow. Animal models have shown that less than 0.01% of circulation tumor cells form metastasis.14 The circulatory system exposes cells to new stresses that they must overcome, including turbulent flow and circulation immune cells. If cells survive here, they must still be able to traverse the endothelium of a new organ site. Once there, they must be able to grow into and through a new organ with distinct extracellular conditions. One can imagine that the extracellular environment in the breast is very different from that found in bone.
Why then do some tumors spread to bone, whereas others do not? This question and the concepts leading up to it are areas of active research. The spine is the most common site of osseous metastasis; Batson proposed that the valveless venous system surrounding the spine was the primary reason for the higher number of bone metastases in the spine.15 This is akin to the embolic theory of metastasis, which Paget had argued was an insufficient explanation for the predilection of some cancers to certain organs.6 Although most researchers agree that embolism and anatomy are secondary to genetic components of metastasis, important anatomic considerations should be kept in mind. Bone marrow sinusoids (capillaries) contain fenestrations that allow the ingress and egress of hematopoietic cells.16 This may contribute to how cancer cells enter the bone.
Entering the bone marrow is not sufficient for macrometastasis to occur. Tumor cells must be able to live and grow within the extracellular matrix of bone. This necessarily requires the breakdown of bone, which is a task that only osteoclasts can accomplish. Some cancer cells produce factors that directly activate osteoclasts (parathyroid-related peptide [PTHrP], interleukin [IL]-11, IL-6), granulocyte macrophage colony-stimulating factor (GM-CSF), and tumor necrosis factor-alpha (TNF-α).17–20 GM-CSF directly stimulates osteoclastogenesis. PTHrP, IL-11, IL-6, and TNF-α all stimulate osteoblasts to produce receptor activator of nuclear factor-κB ligand (RANKL), which stimulates osteoclast formation. Expression of cytokines that stimulate osteoclasts may not be unique to cancers that enter the bone marrow sinusoids; however, their production may offer a survival advantage only in areas rich in osteoclasts. Therefore, these cells are selected to grow in bone, rather than other cells that may accompany them into the marrow. These same cells may not thrive in the liver or lung, where other cytokines offer a survival advantage.
The contribution of the molecular mechanisms behind metastasis is clear. However, the vascular contribution to metastasis still has merit. The valveless venous system surrounding the axial skeleton and parts of the pelvis are subject to stagnation, particularly during episodes of increased intra-abdominal pressure, such as during Valsalva.15 Additionally, larger bones have more area in which metastatic cells can accumulate and a corresponding larger blood supply, delivering more cells.
When one considers the pathophysiology of metastatic bone disease, the gross biomechanical properties of metastatic lesions must be considered. When a lesion is seen on plain radiographs, the biomechanical properties of the affected bone are not equivalent to those of normal pre-metastatic bone. This is clear, but how much does the lesion affect the structural quality of the bone? Hipp and colleagues determined that both lytic and blastic metastases weaken bone, but lytic lesions weaken bone to a greater extent. Lytic lesions have a greater impact on the strength and stiffness of bone through their disruption of mineral, organic, and structural components of bone. Blastic lesions disrupt the trabecular framework of bone; this is detrimental to overall stiffness and fatigue properties, while sparing bone strength.21 Lytic lesions in the cortex force an accumulation of stress in the bone surrounding the lesion. This so-called stress riser can lead to a fracture. A lesion that measures 20% of the overall bone diameter decreases the bending strength of bone by 40%.22 However, even small cortical disruptions can significantly decrease the structural integrity of bone. One biomechanical study evaluated the energy-absorbing capacity of bone after drill holes were made in the femoral shaft. The holes measured between 2.8 mm and 3.6 mm, which is roughly 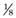 of an inch. Such lesions reduced the energy-absorbing capacity of bone by 55%, leading to torsion-type fractures.23 An “open section” defect describes the situation in which the size of a lesion is greater than the diameter of the bone. This defect reduces bending strength by 90%. However, the femur is most likely to break when a torsional load is applied, as when a patient pivots when rising from a chair.22
of an inch. Such lesions reduced the energy-absorbing capacity of bone by 55%, leading to torsion-type fractures.23 An “open section” defect describes the situation in which the size of a lesion is greater than the diameter of the bone. This defect reduces bending strength by 90%. However, the femur is most likely to break when a torsional load is applied, as when a patient pivots when rising from a chair.22
Clinical Features and Diagnosis
Pain is the most important and most common symptom of bony metastasis. It is the most feared complication of cancer in general, and most people perceive that death from cancer will be painful. Nearly 70% of cancer patients report that severe pain may lead them to consider suicide.24–26 In spite of this, cancer pain was reported to be undertreated by 86% of cancer physicians.27 The orthopedic surgeon has a vital role to play in the management of cancer pain.
Prostaglandins and osteoclast-activating factors sensitize nociceptors and produce hyperalgesia and pain during osteolysis.28 This can produce pain even when the structural integrity of bone remains relatively intact. Patients often complain of a deep aching pain that often prevents them from sleeping. A key part of the history with regard to this pain is that it troubles them even when they are lying down. This is different from pain that occurs only when the patient is ambulating or bearing weight. Pain that is relieved by rest is more likely related to a structural problem and is termed functional or mechanical pain. It is very important to distinguish these two presentations. The first may respond to systemic therapy or radiation therapy, whereas the second will not likely respond to either.
Pain about the hip may be referred from the spine. An L1 compression fracture may compress the exiting nerve roots, causing referred pain to the hip/groin area (Fig. 52-1). Alternatively, knee pain can be the only manifestation of hip pathology. In general, acetabular, femoral head, and neck and pubic rami pathology will manifest as groin pain. Gentle rotation of the femoral head may help distinguish intracapsular pathology from pubic rami pathology. Tenderness over the pubic ramus can also help distinguish these areas. As with other types of hip pain, patients may demonstrate a Trendelenburg gait as they try to unload their hip joint. Groin pain with straight-leg raise or pain with passive rotation of the hip may indicate hip pathology.
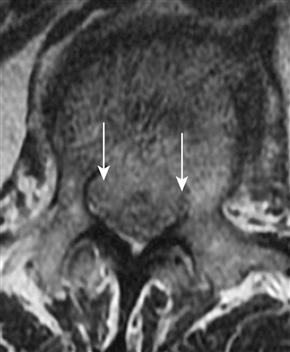
Figure 52-1 This sagittal, T2-weighted magnetic resonance image (MRI) was taken from a patient who described bilateral groin pain. Images of her hips were negative. This pathologic fracture at L1 was causing compression of her exiting nerve roots. Subsequent biopsy revealed metastatic carcinoma.
It is important to assess the patient’s ability to ambulate. It is one of the key features that determine prognosis and treatment options. The inability to ambulate may prevent patients from being considered for systemic therapy or clinical trials. Ambulation is used as a surrogate for overall function. It is important for the orthopedist to help determine whether the patient’s functional capacity is the problem, or whether his/her inability to ambulate is amenable to surgery.
Hypercalcemia of malignancy is the most common paraneoplastic condition in patients with cancer.29 It has been reported to occur in nearly half of patients with metastatic breast carcinoma to bone.30,31 Dehydration is a common problem in patients treated with chemotherapy for metastatic disease. This can exacerbate an underlying hypercalcemia. Increased serum levels of calcium can occur from robust osteoclastic resorption, which is seen with widely metastatic bone disease. Some carcinomas express PTHrP, which causes hypercalcemia as a result of secondary hyperparathyroidism. Patients with hypercalcemia can become lethargic, fatigued, and anorexic. Patients may become comatose and/or may develop cardiac arrhythmia if left untreated.32 Initial management of such patients consists of fluid resuscitation with normal saline. Thiazide diuretics should be stopped because they foster calcium retention. Once the patient has been adequately hydrated, a loop diuretic may be given because it helps clear calcium via the urine.33 In addition, bisphosphonates have been approved for the treatment of hypercalcemia.
Plain radiographs are an important part of the assessment of any bony pain. This is particularly true in patients suspected of having metastatic disease. Lytic or sclerotic lesions are often detected with plain radiographs. However, a normal x-ray does not completely rule out a metastatic focus in that 30% to 50% of bone must be destroyed before it is detected on plain radiographs. Judet views of the pelvis should also be included in the workup of hip pain in patients suspected of metastatic disease, because they provide useful information regarding the anterior and posterior column. If surgery of the hip is planned, then full-length femur x-rays are mandated. It is important to not miss a subclinical lesion in the femur. If such a lesion is not sought, then a short-stemmed prosthesis may be inadvertently placed just above it. If this lesion later fractures, reconstruction becomes more complex owing to the previously placed prosthesis.
When a lesion is detected, the treating physician should ask two questions. Is this lesion primary or metastatic? If this lesion is metastatic, are other lesions present? Bone scintigraphy is the imaging modality best suited to answer this second question.34 Bone scans allow the activity of osteoblasts to be tracked. The radiolabeled diphosphate is taken up by osteoblasts and deposited into hydroxyapatite. Those areas of the skeleton in which osteoblasts are more active will lay down more radioactive tracer, which will be detected in the displayed images. A positive bone scan must be followed by plain radiography because a positive scan is not specific. In addition, a negative bone scan does not necessarily rule out metastasis. Myeloma is notorious for being negative on bone scans owing to its failure to elicit an osteoblastic response. This is likely related to the ability of myeloma to stimulate the expression of RANKL by cells other than osteoblasts.35,36
Radiographs serve as the mainstay for surgical evaluation of metastatic bone disease. However, if radiographs are equivocal, computed tomography is the best modality to further characterize the structural integrity of the bone. It is particularly useful about the hip because it provides useful information about the dome of the acetabulum and the quality of the anterior and posterior column.37 It will also allow better visualization of subtle fractures.
Magnetic resonance imaging (MRI) is another modality that has a place in the analysis of metastatic disease. MRI is most commonly employed for spinal lesions, in which the ability to view marrow infiltration and nervous structures is useful. MRI may be used to detect tumor infiltration of bone marrow (and avoid a biopsy) in a patient with a positive bone scan but normal radiographic and/or computed tomography (CT) imaging. It is also useful in cases of occult fracture when a CT is negative.38 However, in cases of known metastatic disease, plain radiographs and CT are often better tests, in that they provide actionable information in a cost-effective and time-efficient manner. For example, if a patient has known metastasis to the bones and has pain in the femur, it is best to order plain radiographs first. CT scan will provide further information about the structural quality of the bone if the situation remains unclear, and the additional information will influence the decision for surgery. MRI may show marrow disease that is not detected on plain x-ray or CT, but the presence of marrow disease is not particularly helpful in a patient with known metastasis.
When surgery is planned for patients with particularly vascular tumors such as thyroid, renal, and hepatocellular carcinoma, arteriography and embolization is considered to prevent excessive blood loss if the lesion is to be entered at the time of surgery. If wide resection is planned (e.g., a proximal femur metastatic renal cell lesion), embolization may not be necessary.
The decision to proceed with surgery is predicated on the need to treat a fracture or prevent an impending fracture. Predicting which lesions will lead to a fracture is not always easy. Several studies provide useful parameters that one can use as a guide. Lytic disease involving more than 2.5 cm of the femoral cortex has been identified as a means to predict impending fracture in the femur.39 Subsequent studies have used painful lesions eroding more than 50% of the femoral diameter as a benchmark for surgery.40 This criterion was later changed to include painful lesions involving more than 30% of the femoral diameter that have failed radiation treatment.41 Another study added painful lesions involving the subtrochanteric region as at high risk for fracture.42 However, one of the largest studies evaluating more than 516 lesions in 203 patients with metastatic breast cancer could not correlate pain or lesion size with fracture risk.43 In addition, a biomechanical study failed to demonstrate a strong correlation between prediction of fracture risk based on interpretation of plain radiography and CT with actual mechanical strength of bone.44 A follow-up study from the same group found that bone mineral content as assessed by dual energy x-ray absorptiometry (DEXA) could be useful in predicting failure in patients with lytic lesions.45 Subsequent studies have demonstrated that quantitative computed tomography may be useful in predicting fractures.46
In 1989 Mirel proposed a scoring system to predict impending fracture based on four criteria, including lesion location, size (relative to the diameter of the shaft), pain, and radiographic appearance47 (Table 52-1). Mirel’s criteria have since become the most common framework for evaluation of patients with metastatic skeletal disease. Scores can range from 4 to 12. Higher scores predicting fracture were given to lesions about the hip (peritrochanteric), lesions involving more than 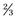 the diameter of the bone, lytic lesions, and painful lesions. Two groups emerged from this study. One group, which did not have a fracture, had a mean score of 7; the other group, which did have a fracture, had a mean score of 10. Significant overlap was noted between the two groups. One third of patients who developed a fracture had a score below 10. The criteria proposed in this study serve as a useful starting point when one is considering a metastatic lesion; however, there are no fully validated criteria on which one can completely base one’s decision. Although means of quantifying fracture risk are improving, a pragmatic approach balancing anticipated load on the hip relative to its load-bearing capacity must be kept in mind. Each patient must be evaluated with this concept in mind, and quantitative assessments must be used as an adjuvant to one’s clinical acumen.
the diameter of the bone, lytic lesions, and painful lesions. Two groups emerged from this study. One group, which did not have a fracture, had a mean score of 7; the other group, which did have a fracture, had a mean score of 10. Significant overlap was noted between the two groups. One third of patients who developed a fracture had a score below 10. The criteria proposed in this study serve as a useful starting point when one is considering a metastatic lesion; however, there are no fully validated criteria on which one can completely base one’s decision. Although means of quantifying fracture risk are improving, a pragmatic approach balancing anticipated load on the hip relative to its load-bearing capacity must be kept in mind. Each patient must be evaluated with this concept in mind, and quantitative assessments must be used as an adjuvant to one’s clinical acumen.
Table 52-1
The Mirel Scoring System
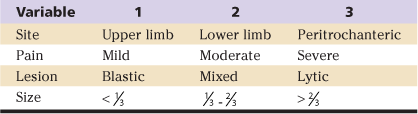
Adapted from Mirels H: Metastatic disease in long bones: a proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res 249:256, 1989.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








