Metal-on-Metal Bearings
Philip A. O’Connor, Brent A. Lanting and Steven J. MacDonald
Key Points
Advantages of Metal-on-Metal Bearings
Disadvantages of Metal-on-Metal Bearings
• Production of metal ions (cobalt and chromium)
• Hypersensitivity reactions and pseudotumors
• High revisions of some prosthesis leading to implant recall
• Contraindicated in patients with renal failure
Introduction
Metal-on-metal (MOM) articulations evolved simultaneously with Charnley’s low-friction arthroplasty (LFA). Inadequate clearances, poor manufacturing tolerances, and poor implantation technique all lead to early failure and the demise of first-generation MOM implants. The resurgence of interest in alternatives to the LFA followed observations of failure secondary to polyethylene wear and osteolysis, particularly in younger, more active patients. After 2 decades, newer manufacturing capabilities and greater understanding of tribology allowed the development of second-generation MOM total hip arthroplasty (THA). In MOM bearings, wear volume has been shown to decrease as femoral head size increases, hence the development of modern resurfacing-type implants, using thinner acetabular components and large-diameter femoral heads. Modular, large MOM bearings offer the potential for low wear rates, greater stability, and less impingement with increased head-to-neck ratios. In 2005, metal-on-metal bearings accounted for 35% of all hip arthroplasties performed in the United States.1 However, recent reports of lymphocytic aggregates, elevated metal ion concentrations, and hypersensitivity reactions2 have raised concerns regarding the safety of MOM bearings. Furthermore, registry data have indicated a revision rate of greater than 10% for some implants within 10 years.3 Implant and radiographic features that contribute to early failure are not completely understood, and only limited short to midterm results are available. Concerns for local and systemic toxicity and elevated revision rates are present in spite of improved wear performance.
Development of Metal-on-Metal Bearings
The wear properties of metal for arthroplasty were recognized as far back as 1938 by Smith-Peterson. It was on the recommendation of his dentist that he turned to Vitallium, a cobalt-chrome alloy, after earlier failures of his “mold arthroplasty.” Several long-term reports on these implants have been put forth, and the longest implant is known to have survived 56 years.4 Philip Wiles of Middlesex implanted the first metallic THA, which was made of stainless steel. The components were ground to fit together accurately. He used his design in 1938 on six patients with Still’s disease but reported very limited success.5 Little evidence remains of his efforts because nearly all radiographs were lost during World War II. Further modifications were made, and in 1957, his new implant was inserted into eight patients—again with limited success. Kenneth McKee had worked as a registrar to Wiles and in Norwich went on to develop his own THA. After a visit to America in 1953, he came across the Thompson prosthesis, which he would then mate with his cast cobalt-chromium cloverleaf socket, which was fixed by screws. Charnley’s use of acrylic cement provided the breakthrough for vastly improved femoral fixation. McKee would later modify the femoral stem, initially by burring down the undersurface of the neck.
Eventually a new design was developed. John Watson-Farrar was registrar to McKee, and it was his suggestion that a larger femoral component should be used. The final version of the McKee-Farrar was produced in 1965. The 38-mm femoral head articulated with a thin cobalt-chromium cup. Several long-term reports highlight the success of this MOM construct, with the longest series reporting nearly 30 years’ follow-up.6 Clinical success was limited, and early component loosening was attributed to poor manufacturing quality, variable tolerance, and failure of fixation.7 Early loosening of the acetabular component, due to equatorial contact, was one of the reasons why this design was abandoned. In Surrey, Peter Ring designed an acetabular component that he mated with a 40-mm Moore prosthesis, and the components were wrapped together. His design was inserted without the use of acrylic cement because the acetabular component had a long thread that was screwed into the ilium. Ring reported one series with survivorship of 85% at 6 to 11 years.8 Varying modification of the implants took place, with an eventual move to polyethylene cups that proved to be inferior.
Weber observed that several Müller-McKee replacements were still functioning after 25 years.9 Implants that did produce long-term survivorship were noted to have little or no wear or incidence of osteolysis.10,11 As a result, he was motivated to re-explore MOM bearing couples, reasoning that low wear and low friction were contributing factors to the longevity of the implants he observed. Failure of the McKee-Farrar implant was due to design deficiencies rather than to accelerated wear, and the low wear rates seen might limit the production of wear particles and the development of osteolysis. At the end of the 1970s, Weber approached Sulzer Brothers Limited (Sulzer Inc., Winterthur, Switzerland) to ask for help in developing a modern MOM couple with better manufacturing precision and tolerances. It was already thought that high carbon content of the cobalt-chromium-molybdenum (Co-Cr-Mo) alloy could provide improved lower wear.9 The Metasul THA (Zimmer, Inc., Warsaw, Ind) produced by Sulzer consisted of a 28-mm or 32-mm modular metal Co-Cr-Mo femoral head and an acetabular metal inlay embedded in the polyethylene cup, with a four-layer sintered stainless steel mesh surface for enhanced cement fixation. In 1988, Weber started to implant the Metasul bearings, commencing the era of second-generation MOM bearings. Although the initial second-generation MOM bearings measured 28 or 32 mm, MOM designs quickly evolved. In a desire to maximize the benefits of increased stability and range of motion, head sizes increased to reach the 40- to 50- mm range. Cup designs included metal inlay liner in a polyethylene cup, hemispherical cups with modular liners, and monoblock, sub-hemispherical acetabular components. Several cup design features resulted in a sub-hemispherical articulation. To increase cup stiffness, the dome was thickened. To allow fluid ingress, the cup edges were rounded. Some cups had speciallized insertional tools that locked within the cup. A sub-hemispherical cup also decreased impingement potential. Griffin and associates investigated 33 cups of different sizes from six companies. Notably, the mean articular arc was 160.5 degrees; with the smallest articular arc being 151.8 degrees.12
Wear
Standard THA with a metal head articulating against a polyethylene couple has yielded highly predictable and reproducible good results. The search for alternative bearings has occurred in part because of observed osteolysis and component failure. In addition, younger patients are now presenting for arthroplasty who will have increased tribiological demands placed on their implants compared with those reported in currently available historic long-term outcome data. Data from the Swedish Hip Arthroplasty Register reveal 21-year survivorship with Charnley’s prosthesis of 81.7% in primary hip osteoarthritis.13 Metal bearings have evolved in hip arthroplasty to meet the challenge of reducing wear-related failure for a more active, younger population with an increased life expectancy. One million cycles in a hip joint simulator has been compared with 1-year prosthetic use by the patient. However, Schmalzried and associates14 reported on a group of 33 patients with well-functioning THAs and noted that the average patient’s walking activity approached 2 million cycles per year, and up to 3.5 million cycles per year in highly active people.15
The most significant advantage of the use of a MOM bearing couple in THA is the clearly documented reduction in wear of the bearing surface.16 Wear portends bearing failure, and rates for MOM articulations have been estimated to be up to 100 times lower than those of metal-on-polyethylene (MOP) couples.16 Polyethylene wear particles induce osteolysis through a well-understood inflammatory cascade.17 Schulte and coworkers measured wear rates of Charnley’s MOP prostheses on radiographs. At 20 years, they reported an average wear rate of 0.074 mm/yr.18 A vast majority of PE wear particles are between 0.1 and 0.5 µm, and particles smaller than 0.5 µm are of the critical size for macrophage activation. Metal ion production can occur with a conventional MOP bearing from the femoral component through corrosion and/or fretting at the head-neck coupling.19 Estimates of wear rates from MOM McKee-Farrar implants retrieved at 20 years were 0.0042 mm/yr, that is, 25 times lower than MOP rates.
Metal bearings exhibit a complex tribology. Wear is dependent on metallurgy, design, geometry, radial clearance, lubrication, loading regimen, kinematics, and component position.20 Cobalt-chromium-molybdenum is the most commonly used alloy for MOM bearings; however, it should be emphasized that metal bearings represent a large heterogeneous group. The wear pattern of MOM bearings exhibits two distinct phases, as demonstrated in laboratory simulator experiments.16,21,22An initial “bedding-in” period is seen for the first million cycles, or the first year in vivo, during which self-polishing of the bearing takes place. The subsequent “steady-state” phase exhibits a lower continuous wear rate. The mean linear wear rate of a MOM hip has been shown to be about 5 µm/yr, and this corresponds to a volumetric wear of 1 mm3/yr.23 This is approximately 40 times lower in linear wear as compared with MOP articulations. Retrieval studies24 of 118 Metasul MOM bearings documented a linear rate of wear of 5 µm/yr, which is at least 20 times less than that of MOP. The volumetric rate of wear of 0.3 mm3/yr is at least 60 times less than that of MOP (17.9 mm3/yr). Langton looked at 15 Articular Surface Replacement (ASR) hips (Depuy Orthopaedics, Inc., Warsaw, Ind) explanted at a mean of 18 months, and demonstrated a mean linear wear rate of 2.9 µm.25 Increased variation was reported by Schmidt and coworkers on 11 McKee-Farrar hips explanted at a mean of 16.3 years, with linear wear rates ranging from 0.1 to 300 µm/yr.26 Witzleb et al reported higher levels of volumetric wear rates in 10 Birmingham Hip Resurfacing (BHR) hips (Smith & Nephew Orthopaedics, Memphis, Tenn) explanted at a mean of 13 months, with volumetric wear rates of up to 27 mm3/yr.27 Component type and size and position all play important clinical roles that are not been fully described in a laboratory setting, which may result in in vivo differences in wear rates.
Wear rate has been implicated in the development of pseudotumours. Higher wear rates and greater prevalence of edge wear have been demonstrated in a group of 18 patients with resurfacings revised for pseudotumor compared with 18 patients revised for other reasons.28 Volumetric wear (3.3 ± 5.7 mm3/yr) was six times greater and the linear wear rate (8.4 ± 8.7 µm/yr) was three times greater for the pseudotumor group than for the control group. The argument that cup position is correlated to higher wear due to edge loading as measured by ion levels was re-enforced by a recent paper that examined MOM revisions for pseudotumors, with revisions for other reasons as a control group.29 In this article, thirty resurfacing hips were retrieved—8 for pseudotumor formation—and were assessed with a roundness machine. Those hips that had a pseudotumor were found to have a higher rate of wear of the femoral component (8.1 µm/yr [range, 2.75 to 25.4 µm/yr]) than those that did not have a pseudotumour (1.8 µm/yr [range, 0.82 to 4.14 µm/yr]). Acetabular component pseudotumour revisions also showed increased wear, at 7.36 µm/yr (range, 1.61 to 24.9 µm/yr), versus 1.28 µm/yr (range, 0.81 to 3.33 µm/yr). Wear patterns were consistent with edge loading.
Metallurgy
Carbon carbides are produced when carbon reacts with metal at high temperatures. Heat treatment (annealing) of high-carbon (0.266 wt %) Co-Cr-Mo alters the size and percentage of carbides on the bearing surface, allowing the hard carbides to diffuse into the softer matrix material. Single-treatment annealing leads to smaller carbides in the material, whereas double-treatment annealing leads to almost no visible carbides under scanning electron microscpy (SEM). Kinburn examined the effects of carbides on wear in three different as cast high-carbon components.30 Their results indicate that “heat treatment by solution annealing of a Co-Cr-Mo material significantly increased the wear rates.” Dowson performed a hip simulator study to compare various combinations of 36-mm, high- and low-carbon, wrought and cast Co-Cr-Mo femoral heads.31 The low-carbon material exhibited higher wear than the high-carbon wrought material. No significant difference was noted between wrought and cast high-carbon materials. High-carbon/high-carbon pairings show the lowest wear rates in hip joint simulator tests.32
The ability to establish fluid-film lubrication is a major factor in the very low wear rates seen in MOM hips. Head diameter and diametrical clearance are central to this concept. The wear rate of MOM is reduced with a larger head size in combination with lower clearances; this is in contradistinction to MOP bearings. In Charnley’s LFA, wear was shown to be proportional to the sliding distance. By reducing the femoral head diameter, he aimed to reduce wear volume. Charnley chose a femoral head diameter that was half the diameter of the polyethylene socket. In contrast, lubrication analysis of metal bearings reveals that as the head size increased, the potential to achieve fluid-film lubrication also increased, resulting in reduced wear.33 Dowson has shown that as diametrical clearance increased, both bedding-in wear and steady-state wear increased significantly.31 So the most advantageous design of an MOM bearing includes low clearance and a larger diameter. The challenge with lower clearances, in particular with a monoblock design, is that cups can deflect on insertion, which can potentially lead to equatorial binding.34 Additionally, manufacturing tolerances are a factor in the ability to effectively reduce clearances, once again because of the concern of too small a clearance, leading to equatorial binding.
Metal Particles
Metallic debris particles are in the order of 10 to 50 nm in size.35 However, the volume of metallic particles produced is much greater. Corrosion of particles, on exposure to periprosthetic fluids, releases metal ions into the surrounding tissue. Subsequent dissemination of chromium (Cr) and cobalt (Co) ions throughout the body has raised concerns about elevated serum, urinary, and tissue levels. Doorn and colleagues36 were able to estimate the total number of particles produced per year in vivo because wear volumes of the prostheses and the particle size had been determined. They estimated that from 6.7 × 1012 to 2.5 × 1014 metal particles were produced per year in three different patients with MOM THAs. This figure is 13 to 500 times greater than that of the 5 × 1011 ultra-high-molecular-weight polyethylene (UHMWPE) particles produced in a typical MOP prosthesis. Production of metallic debris has raised concerns regarding metal ion levels and tissue hypersenstivity reactions, the biological effects of which remain uncertain.
Metal Ion Analysis
MOM THA wear, with production of metal particles, has led to concern for systemic exposure to metal ions. In 1973, Coleman37 reported analytical techniques for the measurement of cobalt and chromium in blood and urine in a series of 12 patients undergoing THA, 9 of whom had MOM bearings and 3 of whom had MOP bearings. Study results showed an almost threefold elevation of chromium in whole blood, an 11-fold increase in cobalt, a 15-fold increase in chromium in urine, and a 48-fold cobalt increase in urine in patients with MOM components. Elevated systemic ion levels has been used as a marker for localized pathology, with Bosker and associates reporting that patients with elevated serum ion levels were at a four times higher risk of developing pseudotumor. 38 Also, systemic toxicity has been reported secondary to elevated ion levels.39,40 This has led to increasing interest in the measurement and monitoring of ion levels of patients with MOM hips.
Lazennec and coworkers analyzed the metal ion concentration of patients in their cohort. Sampling was performed at 3 months, 6 months, 1 year, and every year postoperatively until the last endpoint was reached. Chromium and cobalt levels were assayed in plasma using inductively coupled optical emission spectrometry (ICP-OES).41 Median serum cobalt levels at 9 years were 1.55 µg/L with little variation observed over time. Serum chromium levels at 9 years were 1.49 µg/L and showed a slight decrease over time. In eight patients who underwent revision for aseptic loosening, the median cobalt was almost 17 times higher at 26 µg/L. Investigators report one revision case for persistent and unexplained pain with well-fixed implants. Grossly elevated serum cobalt levels were detected, and at operation, massive and macroscopic metallosis was observed in the joint. The patient’s symptoms settled after exchange of bearing surfaces with ceramic-on-ceramic. In an additional study, it has been seen that after revision, metal ions significantly decrease.42 Pre-revision cobalt was 307.1 nM/L (range, 25 to 2300 nM/L), and chrome was 204.5 nM/L (range, 25 to 850 nM/L). After revision, cobalt levels decreased to 6.6 nM/L (range, 1.7 to 23.8 nM/L), and chrome levels to 67.3 nM/L (range, 19 to 885 nM/L). In this study, one patient with persistently elevated ion levels after revision was awaiting revision for a contralateral MOM THA.
Several published series of second-generation MOM total hip systems have shown elevated cobalt and chromium levels in blood and/or urine. These show a fivefold to 10-fold increase from preoperative to postoperative blood cobalt values (average preoperative blood cobalt level, 0.15 µg/L; postoperative level, 1.0 µg/L).43 MacDonald and coworkers44 performed a prospective, randomized, blinded clinical trial in 41 patients to evaluate MOM versus MOP THAs. Patients were assessed preoperatively and postoperatively using erythrocyte metal ion analysis of cobalt, chromium, and titanium (Co, Cr, Ti), as well as urine metal ion analysis (Co, Cr, Ti). Patients were followed for a minimum of 2 years. Those who had MOM inserts had on average a 7.9-fold increase in erythrocyte cobalt, a 2.3-fold increase in erythrocyte chromium, a 1.7-fold increase in erythrocyte titanium, a 35.1-fold increase in urine cobalt, a 17.4-fold increase in urine chromium, and a 2.6-fold increase in urine titanium at 2 years’ follow-up. Patients receiving a polyethylene insert had no change in erythrocyte titanium, urine cobalt, or urine chromium and a 1.5-fold increase in erythrocyte cobalt, a 2.2-fold increase in erythrocyte chromium, and a 4.2-fold increase in urine titanium. Forty-one percent of patients receiving metal-on-metal articulations had increasing metal ion levels at the latest follow-up.
Although it is known that ion levels rise after MOM THAs compared to those in control patients, and that elevated ion levels may be indicative of pathologic wear, challenges to the interpretation of serum ion levels are known. Results from patients that have had bilateral MOM articulations are more difficult to interpret because ion levels are greater than, but not double, that of unilateral cases.45 Also, serum ion levels increase with increased activity of the patient.46 Finally, differences between MOM THAs and resurfacings have been identified, limiting the ability to extrapolate knowledge. A meta-analysis was conducted that reviewed comparative studies of MOM total versus resurfacing hip arthroplasties.47 This study was limited by the relatively small number of subjects, and of large head MOM THA cases. Only metal cobalt levels only were found to be lower in the resurfacing group, and it was found that chrome levels were not affected.
Problems with comparison of published data on metal ion concentrations are due to lack of uniformity. Specimens analyzed in publications include whole blood, serum, erythrocytes, urine, synovial fluid, periprosthetic tissue, and remote tissue. Blood and urine are the most commonly assayed samples. Guidelines on standardized methods for sampling, storing, processing, analyzing, and reporting have been established.48 Retrieval analysis24 has provided important data on wear in MOM bearings, but surrogate markers of wear in MOM hip arthroplasties are needed, because it is not possible to measure wear radiographically.49 Serum metal levels can provide some comparative information on the performance of MOM hip arthroplasty systems as long as patient demographics, activity level, and renal function, as well as analytical technique used are comparable.49 Finally, the effect of time of exposure is not understood. Griffin and colleagues found that ion levels did not correlate with extent of soft tissue damage, but the time in situ did have statistical significance.50 However, significance is lost once ion levels and time in situ are combined. Bernstein and coworkers reported on 163 Metasul 28-mm MOM hips at 8.9 years (range, 7-13 years) and noted that cobalt levels peaked at 4 years at 2.87 µg/L, subsequently decreased to 2.0 µg/L after 9 years.51 Chrome levels increased to five years to 0.75 µg/L and then decreased after 7 years to 0.56 µg/L. Griffin and associates describe an increase in soft tissue damage with increased time in situ with a time to revision of less than 5 years; therefore, it is unknown whether soft tissue damage is due to time in situ or to steadily climbing ion levels.50 However, the potential for increased soft tissue damage with increased time in-situ needs to be considered.
Healthcare regulatory bodies such as the MHRA recommend following serum ion levels in patients with MOM articulations. However, the impact of elevated ions levels is not well understood. A safe level of metal ion concentration has yet to be defined for patients with MOM hip arthroplasties, and to date, only a fraction of the estimated 600,000 MOM hip arthroplasties implanted have undergone metal ion analysis. Although serum ions levels may provide clinical information about the wear rate of the MOM articulation and potential for sub-optimal function,45 absolute values do not provide information on the extent of soft tissue destruction found intra-operatively.50 This finding was corroborated in another recent paper, which recommended MRI cross-sectional imaging for MOM follow-up.52
Component Position
Higher wear rates have been reported with acetabular components inserted with a cup inclination greater than 50 degrees in MOP bearings.53 Brodner examined three patients with cup inclinations of 58 degrees, 61 degrees, and 63 degrees who had serum chromium levels of 10.4 µg/L, 33.6 µg/L, and 12.1 µg/L, and serum cobalt levels of 4.9 µg/L, 26.8 µg/L, and 12.9 µg/L. The overall study group had a median chromium concentration of 1.1 µg/L and a median cobalt level of 0.5 µg/L. Although Brodner and associates reported that no statistical difference in correlation could be detected between three groups of varying cup inclination, multiple other authors did find this difference.54 Angadji and colleagues55 examined the effect of cup inclination on MOM bearings in vitro using six MoM bearings with a head diameter of 40 mm. Three different angles of cup inclination were examined: 35 degrees, 50 degrees, and 60 degrees. Higher wear rates were seen with steeper inclination during steady-state wear. As cup inclination increased, the observed wear pattern progressed from polar to the cup rim. Williams and colleagues56 performed a hip joint simulator study to examine the role of increased cup inclination and wear in MOM bearings. Cups placed at 55 degrees showed a fivefold increase in steady-state wear over cups placed at an angle of 45 degrees. The wear pattern at a higher inclination was noted to move toward the superior edge of the cup. DeHaan and associates57 reported a statistically significant increase in blood chromium and cobalt levels with steeply inclined cup position. Mean values for cobalt were 9.8 µg/L (range, 0.6 to 111.3 µg/L; 95% confidence interval [CI], 4.4 to 15.1) for components at greater than 55 degrees, and 2.4 µg/L (range, 0.4 to 31.5 µg/L; 95% CI, 1.8 to 2.9) for components at less than 55 degrees. Mean values for chromium were 9.7 µg/L (range, 0.6 to 94.6 µg/L; 95% CI, 5.3 to 14.1) for components at greater than 55 degrees, and 3.6 µg/L (range, 0.2 to 32.2 µg/L; 95% CI, 2.8 to 4.3) for components at less than 55 degrees of abduction.
Similarly, work by Langton and colleagues58 confirmed a positive correlation between the inclination of the acetabular component and concentrations of cobalt (r = 0.439; P < .001) and chromium (r = 0.372; P = .011). This finding was supported by Hart and colleagues, who found an increase in ion levels with cup inclination of greater than 50 degrees; albeit in a small patient number.59 An additional study revealed similar findings, using direct measurement of wear in 45 retrieved resurfacings.60 Using 5 µm as the cutoff between low and high wear resurfacings, it was demonstrated that low-wearing hips were within 30 to 50 degrees of abduction twice as often as the higher-wear hips. Acetabular and combined version was only weakly correlated with increased wear. However, the impact of patient-specific biomechanics cannot be disregarded. In a group of four subjects, motion pathway, computed tomography (CT) imaging and finite element analysis (FEA) were analyzed by Mellon and associates.61 Based on gait cycle and stair descent motion analysis, cup position as measured by CT, and published hip contact forces, FEA was conducted for 1-degree intervals of cup inclination. This group demonstrated that cup position should not be considered in isolation, and that patient motion patterns do influence the potential for edge loading.
Optimal cup position is a combination of coronal and sagittal plane positioning. It can be seen that a frontal plane cup position of greater than 50 degrees should be avoided. In hip resurfacing components, the recommended lateral opening angle should be between 40 degrees and 45 degrees, and combined anteversion (acetabular and femoral) should be 20 degrees to 30 degrees.62
Clinical Outcome (Table 72-1)
A 10-year outcome of the Metasul MOM bearing was published by Eswaramoorthy.63 A cemented Stuehmer-Weber polyethylene acetabular component with a Metasul bearing was used in 52 hips, and an Allofit uncemented component with a Metasul insert was used in the other 52 hips. The cohort consisted of 100 patients undergoing 104 hip arthroplasties. In all, 15 patients had died before their 10-year review, and all deaths were reported to be unrelated to their well-functioning THAs. Three patients were lost to follow-up; this left 82 patients (85 hips) as the study group. Six revisions were performed, only one of which was done for confirmed infection. One revision was performed in another jurisdiction, and no details were available. Three preocedures were performed for pain, of which two had histologic changes typical of aseptic lymphocytic vasculitis–associated lesion (ALVAL). The reason for revision in the sixth case was presumed infection, although no organism was cultured. Survivorship of the Metasul MOM bearing in this series was 94% at 10 years.
Table 72-1
Clinical Results of Second-Generation Metal-on-Metal Total Hip Arthroplasties
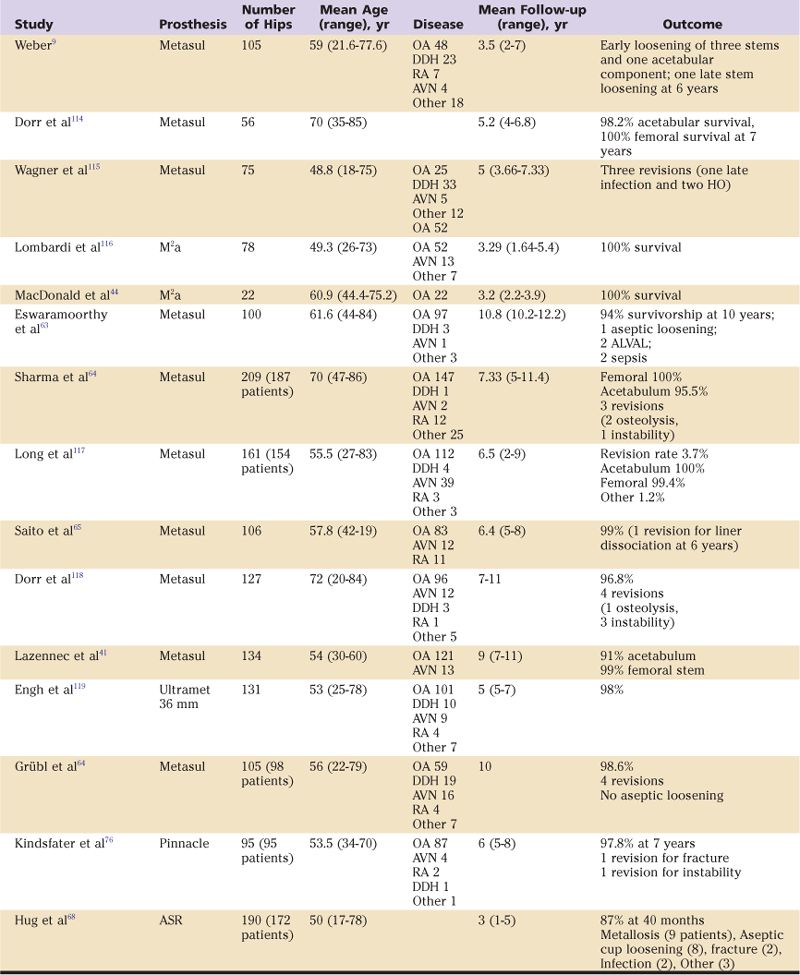
ALVAL, Aseptic lymphocytic vasculitis–associated lesion; AVN, avascular necrosis; DDH, developmental dysplasia of the hip; HO, heteropic ossification; OA, osteoarthritis; RA, rheumatoid arthritis.
Modified with permission from MacDonald SJ, Mehin R: Metal on metal: clinical results with modern implants. Semin Arthroplasty 14:123–130. Copyright © 2003, with permission from Elsevier.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








