CHAPTER 52 Lumbar Nucleus Replacement
Parallel to the development of lumbar artificial total disc replacement (TDR), physicians and researchers have worked on the development of lumbar nucleus replacement.1,2 TDR and nucleus replacement are part of disc arthroplasty, which is intended to provide an alternative to fusion for patients with discogenic back pain and sciatica. Although both procedures preserve index segment motion and reduce the stress on the adjacent motion segments, there are major differences between TDR and nucleus replacement, as follows: (1) Nucleus replacement is more tissue-preserving than TDR. (2) Nucleus replacement technology is simpler and theoretically easier for restoration of normal biomechanical functions than TDR because of the preservation of anulus and ligaments. (3) Nucleus replacement does not affect effective conversion to TDR or fusion if it fails for any reason. (4) Because of the smaller dimension of a nucleus replacement device than a TDR device, the nucleus replacement can be performed with multiple surgical approaches, including the traditional posterior approach, whereas TDR currently has to be performed via an anterior or anterolateral approach.
Clinical Challenge of Nucleus Replacement
Design and Material Options of Nucleus Replacement
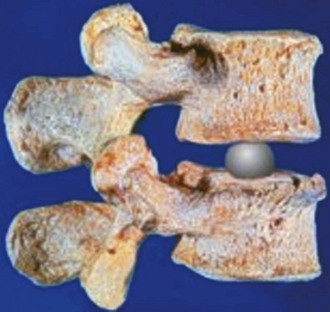
In contrast to the predominant ball-and-socket design with either metal-on-metal or metal-on-polyethylene for the current generation of TDR, the design and material options for nucleus replacement are quite variable. This variability is largely due to the fact that nucleus replacement preserves more of the anatomy of the motion segment, such as anulus and endplates, and more function. The first nucleus replacement with long-term clinical experience was the Fernstrom ball made of stainless steel (Fig. 52–1).3,4 Although this design offered bipolar articulation with the endplates to provide segment motion, it caused high contact stress because of point loading and implant subsidence.
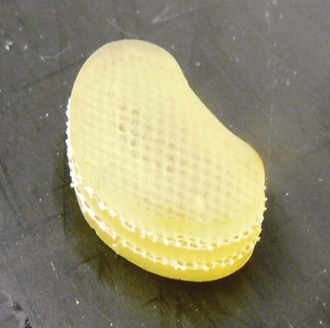
Aquarelle developed by Howmedica (later Stryker) was the first preformed polyvinyl alcohol hydrogel used for nucleus replacement.5 Subsequently, several other preformed hydrogel materials have been used for nucleus replacement, including prosthetic disc nucleus (PDN) (later redesigned and named HydraFlex) (Fig. 52–2) by RayMedica6 and NeuDisc by Replication Medical (Fig. 52–3).7 PDN and NeuDisc used the same type of base hydrogel, an acrylic copolymer hydrogel (HYPAN), with PDN having the hydrogel core encased in a polyethylene jacket and NeuDisc having the hydrogel sandwiched in between the Dacron knitted meshes. The main advantage of using preformed hydrogel materials for nucleus replacement is that they not only mimic the viscoelastic and physiologic properties (imbibing and releasing water during the cyclic loading) of the nucleus, but also they have the ability to bear the mechanical load.
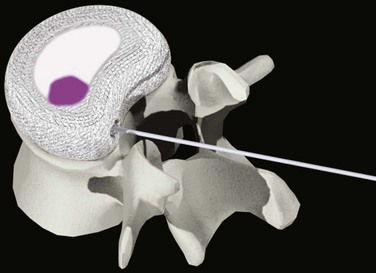
In addition to preformed hydrogel, some in situ curable hydrogel materials have also been used for nucleus replacement. Examples in this category are NuCore (Spine Wave)8 and BioDisc (Cryolife).9 NuCore is an injectable synthetic recombinant protein hydrogel, which is a sequential block copolymer of silk and elastin, with two silk blocks and eight elastin blocks per polymer sequence repeat (Fig. 52–4). The material has a water content and modulus similar to that of the natural nucleus. BioDisc is a biopolymer consisting of a protein-based hydrogel. During implantation, the dispensing device mixes the two components of predetermined ratio, and the glutaraldehyde cross-links the bovine serum albumin (BSA) molecules to each other and to the surrounding tissues.
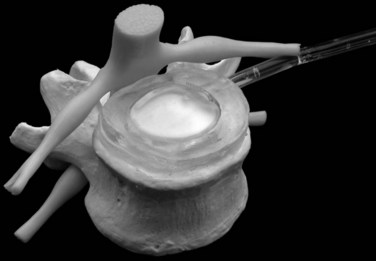
DASCOR (Disc Dynamics) is the most clinically advanced in situ cured nonhydrogel elastomer nucleus replacement and is the only injectable nucleus device with a balloon for containment (Fig. 52–5).10 The injectable polymer is a two-part in situ curable polyurethane. The balloon is also made from polyurethane to facilitate adhesion between the curable polymer and the balloon. There are several clear advantages of using the balloon. First, it allows the polymer to be injected under certain pressure to fill the disc cavity completely without the risk of polymer leaking through the defected anulus. Second, the balloon prevents the direct contact between the uncured or semicured polymer and the surrounding wet tissue, so it avoids the leach of uncured monomers. The isolation of body fluid from the uncured polymer also can ensure better polymerization and a more consistent and stronger mechanical property for the final implant.
Another in situ curable nucleus replacement device is Percutaneous Nucleus Replacement (PNR; Trans1).11 The PNR consists of two threaded titanium vertebral body anchors connected by a cylindric silicone rubber membrane. The two titanium anchors are fixed to the adjacent vertebrae via a trans-sacral approach, and the in situ curable silicone rubber is injected through the sacral anchor into the silicone membrane until the disc cavity is filled.
Some nonelastomeric materials have also been used for nucleus replacement based on the favorable clinical data of the Fernstrom ball. The main advantages of using these nonelastomers for nucleus replacement are their better mechanical strength and durability. The design of the Regain (Biomet) is similar to a modified Fernstrom ball and made of pyrolytic carbon (Fig. 52–6). Although the endplate contacting surfaces of Regain are still convex, they have a much larger radius so that the device has a larger initial contact area and smaller initial contact stress than the Fernstrom ball. Because of the mismatch of the surface contour between the implant and endplates, some subsidence is still inevitable.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree