CHAPTER 46 Lumbar Disc Herniations
Lumbar disc herniations are a common manifestation of degenerative disease.1–3 They tend to occur early within the degenerative cascade, representing the tensile failure of the anulus to contain the gel-like nuclear portion of the disc. With improvements in advanced imaging techniques, lumbar disc herniations have been increasingly recognized in symptomatic and asymptomatic individuals.4
Treatment decision making for patients with herniated discs can be challenging. Nonoperative treatment can be effective in most cases.5–9 Other authors have indicated that surgery leads to superior results, especially in short-term pain relief.1,7–10 Several authors have highlighted the influence of fragment location and pattern and social and psychological factors on outcomes.7–9,11–13 The exact natural history and complex interaction of biologic, psychosocial, ergonomic, and cultural variables have not been well established.
Pathoanatomy
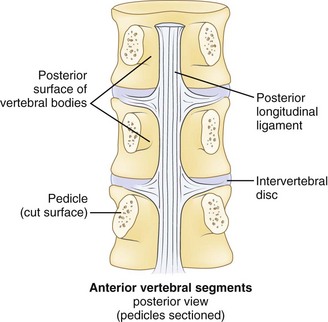
Effective evaluation is based on an intimate understanding of the relationship of the lumbar intervertebral disc to its surrounding structures. The disc is the anterior border of the spinal canal at the facet joint level. It is covered by the thin posterior longitudinal ligament, which is concentrated in the midline, from which small bands extend laterally to cover the inferior aspect of the disc (Fig. 46–1). This configuration leaves the superior part of the posterolateral disc bare and is thought to contribute to the fact that posterolateral (or paracentral) herniations are the most frequent location for herniations to occur. Cumulative degenerative changes occur in this region of the disc from concentration of torsional, axial loading, and flexion-induced biomechanical strains.
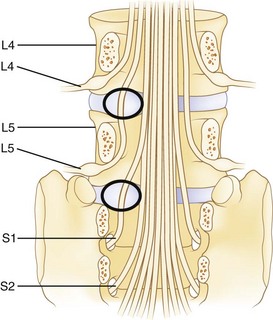
The spinal cord ends at approximately the L1 level in adults to form the conus medullaris. The cauda equina is located within the lumbar spinal canal. It contains the lumbar and sacral nerve roots bathed in cerebrospinal fluid contained, or encapsulated, by the pia, arachnoid, and dural membranes (meninges). Nerve roots branch from the cauda equina one level above their exiting foramen (Fig. 46–2). The L5 nerve root leaves the cauda equina approximately at the level of the L4 vertebral body. It descends inferolaterally to pass anterior to the L4-5 facet joint and posterior to the L4-5 disc. Intimately associated with the inferomedial aspect of the L5 pedicle, the root turns lateral to enter the L5-S1 intervertebral (neural) foramen just proximal to the L5-S1 disc. Within the foramen, sensory cell bodies form the dorsal root ganglion. The root, now termed a postganglionic spinal nerve, exits the neural foramen where it is in close proximity to the lateral aspect of the L5-S1 disc. Fibrous bands (called Hoffman ligaments) often tether the nerve to the disc in this region.14,15 After a short extraspinal course, the nerve divides into a ventral and dorsal primary ramus.
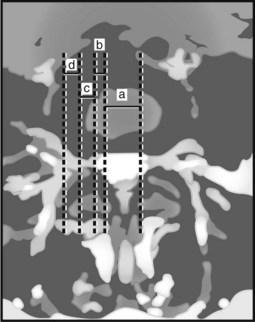
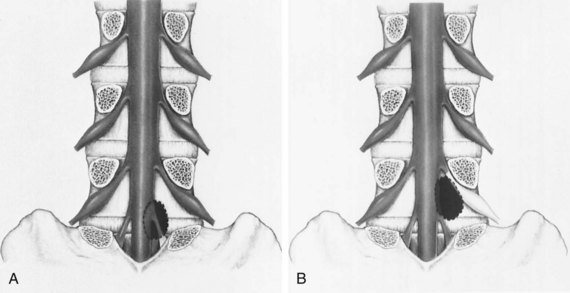
The location of the disc herniation determines which root is primarily affected. The spinal canal can be divided into longitudinal zones (Fig. 46–3). The central zone is delineated by the lateral borders of the cauda equina. The lateral recess is between the lateral border of the cauda equina and the medial border of the pedicle. Although the term lateral recess is frequently used to describe stenosis from bony encroachment (lateral recess stenosis), it sufficiently describes the location of paracentral, posterolateral, or juxtacentral herniations. Within the lateral recess, fragments medial to the nerve root, interposed between it and the cauda equina, are termed axillary herniations (Fig. 46–4). The foraminal zone is between the medial and lateral borders of the pedicle. Herniations beyond the lateral border of the pedicle are within the far-lateral or extraforaminal zone. Herniations in the foraminal or extraforaminal zones usually affect the exiting nerve.
Pathophysiology
Disc Degeneration and Herniation
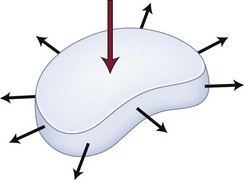
Disc herniation is one stage of the lumbar degenerative cascade. It is considered one of the earlier stages, following internal disc disruption. Herniation occurs through a tear in the anulus fibrosus. The anulus is the thick outer layer that normally withstands tensile forces transferred from the compressed nucleus pulposus (Fig. 46–5).16,17 Force transfer works only if the nucleus-anulus-endplate complex acts as a closed volume system.18 Normally, compression across the disc space leads to increased pressure within the nucleus. The soft nucleus deforms and flattens, pushing against the annular fibers, which then generates tensile hoop stresses. The circumferential fibers are placed under tension, dissipating stresses and containing the anulus.
With disruption of the anulus, the soft nucleus can be pushed through (i.e., herniated) if placed under sufficient pressure. The nucleus must be fluid, or “dynamic,” enough to permit herniation to occur. Discs in younger individuals that have a well-hydrated nucleus are more likely to herniate. Older patients with desiccated discs are less prone to herniation. The ejected portion is typically a fibrocartilaginous fragment.19 In some cases, a piece of anulus or endplate fibrocartilage can be associated with it. In juveniles, an apparent herniation may represent a Salter type II fracture of the vertebral ring apophysis with its attached anulus.
When a portion of the nucleus is ejected, disc mechanics are altered. Frei and colleagues17 showed that nucleotomy alters the loading pattern across the disc space, with the anulus sustaining higher compression forces than normal. This situation can lead to increases in endplate pressures along the periphery where the anulus attaches to the bone. Chondro-osseous metaplastic changes such as osteophytes or sclerosis in these regions are a response to long-standing abnormal loading patterns.
Postural variations can influence intradiscal pressures. The highest pressures have been recorded in patients with the torso forward flexed with weight in hand. In an elegant biomechanical study, Wilder and colleagues16 found that combined lateral bend, flexion, and axial rotation with 15 minutes of exposure to vibration can lead to tears extending from the nucleus across the anulus. This finding may have significance for occupations with exposure to long periods of vibratory stimuli, such as truck drivers and machine workers.
Disc Herniation and Sciatica
In animals and humans, pure compression of a noninflamed nerve produces sensory and motor changes without pain, whereas pain is elicited with manipulation of inflamed nerves.20 These findings suggest that herniated discs large enough to cause mechanical compression of a nerve root may produce focal deficits, but that associated sciatic-type pain is produced only if the nerve root is concurrently irritated or inflamed. Inflammation may be produced by prolonged neuroischemia of the microvasculature of the nerve root from mechanical compression or by nonmechanical, possibly biochemical, factors. This phenomenon helps explain why some patients with small bulges or protrusions contacting inflamed nerves have pain that does not seem to be consistent with the “small” degree of neural compression. Additionally, these patients frequently do not have demonstrable sensory or motor deficit.
Neurochemical factors also have a role in the production of sciatic pain. This role may be related to initiation of an immune response locally or systemically or both. Spiliopoulou and colleagues21 examined IgG and IgM levels in discs excised from patients with sciatica and controls. Although IgG levels were equivalent, elevated levels of IgM were found in discs from sciatica patients but not in controls suggesting a local and humoral antigenic inflammatory reaction as a contributor to pain. Other investigators have shown the role of cytokines in the mediation of root pain. Olmarker and Rydevik22 studied the effects of selective inhibition of tumor necrosis factor-α in a herniated disc model in pigs. They found preservation of nerve conduction velocity and decreased nerve root injury in treated animals versus controls, suggesting a role of tumor necrosis factor-α in potentiating nerve dysfunction.
Similarly, research has suggested that matrix metalloproteinase, nitric oxide, prostaglandin E2, and interleukin-6 in discs excised from patients with herniation and radiculopathy may have a causative role in pain production.23 A more recent investigation was unable to confirm the presence of these inflammatory markers in the epidural space of patients with symptomatic disc herniations, however.24 Other investigators have shown that in extruded or sequestered discs a cellular inflammatory reaction may be locally mediated via T cells and macrophages25; this has been postulated to play a role in herniated disc regression.26
There is evidence of systemic inflammatory responses to disc herniations as well. Brisby and colleagues27 detected elevated levels of glycosphingolipid antibodies in the serum of patients with sciatica and disc herniation compared with healthy volunteers. Elevations were equivalent to those found in patients with autoimmune neurologic disorders such as Guillain-Barré syndrome. Brisby and colleagues27 suggested that a systemic autoimmune response to disc tissue may result in damage, or alteration, of nerve tissue. After age 12, the endplate apophyseal vessels close, which may facilitate an amnestic antigenic response to exposure to extruded nucleus pulposus tissue. These findings are helpful in considering patients who have severe sciatic pain with minimal mechanical compression and patients who seem to have persistent symptoms despite surgical decompression.
Disc Herniation and Back Pain
The concept of vertebrogenic pain has also been suggested. Jinkins and colleagues28 studied the contribution of anterior disc herniations to back pain. They believed that the pain was neurally mediated through branches of the ventral ramus and paravertebral autonomic plexus. Because the herniations were outside the spinal canal, they were not associated with compression of the cauda equina or nerve roots, but most patients complained of lower extremity paresthesias, mostly bilateral, in addition to low back pain. A direct causal relationship between anterior disc herniations and leg symptoms has not been clarified.
Classification of Disc Herniations
Classification of any disorder should be based on identifiable features that have some influence on prognosis or treatment decision making. Many classification systems have been proposed for lumbar disc herniations, although none are all-inclusive or ideal.29,30 It is more appropriate to consider them as tools to describe the herniation.
Morphology
Disc herniations can be described by their morphology. Before the introduction of advanced imaging, morphology was difficult to assess preoperatively. Currently, magnetic resonance imaging (MRI) and to a lesser extent computed tomography (CT) can differentiate disc morphology with reasonable reliability. Spengler and colleagues13 divided herniations into three types (Fig. 46–6). A protruded disc was defined as eccentric bulging through an intact anulus fibrosus. An extrusion was defined as disc material that crosses the anulus but is in continuity with the remaining nucleus within the disc space. A sequestered disc represents a herniation that is not continuous with the disc space; this is the typical “free fragment.”
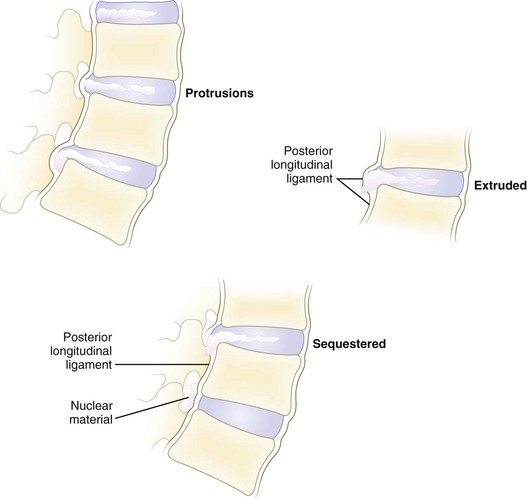
FIGURE 46–6 Classification of disc herniations as described by Spengler and colleagues.13 Disc protrusion is defined as bulging displaced nucleus that has not extended beyond limits of anulus fibrosus. Extrusion extends beyond anulus fibrosus but is still in continuity, at least partially, with parent disc. Sequestered disc herniation implies that fragment has broken free (i.e., free fragment) and is no longer in continuity with parent disc. In some cases, in which disc herniation lies immediately behind vertebral body, it is difficult to tell from which disc the herniated fragment originated.
Other authors have classified discs as either contained or uncontained.31 Contained disc herniations are subligamentous. It is presumed that they have not passed beyond the limits of the posterior longitudinal ligament or the outer layer of the anulus. Uncontained disc herniations have crossed this boundary. Advocates of this system describe contained and uncontained extrusions, with the former remaining beneath the outer layers of the anulus.31
Location
Herniations can be described topographically according to anatomic location (see Fig. 46–3). The herniation can be located within the central zone, lateral recess, foraminal, or extraforaminal regions. Herniations can also exhibit cranial or caudal migration in relation to the disc space.
Timing
Lumbar disc herniations can be organized according to the time from initial symptom onset. These may be arbitrarily divided as acute or chronic. Acute herniations are present for less than 3 to 6 months, whereas chronic discs cause symptoms for a longer time. Breakdown according to this time frame is based on the authors’ sense of what is a reasonable cutoff point. Because the results of disc excision seem to be influenced by the timing of surgery, this categorization is important. From a survey of the literature, it seems that the results of disc excision are compromised if delayed more than 2 to 16 months from symptom onset.12,32–34
History and Symptoms
Many patients describe a prodromal history of long-standing mild to moderate back pain. Although trauma is not the only component leading to a disc herniation, some patients describe a specific incident attributable to the onset of leg and back pain. This incident may be a fall, a twist, or lifting of a heavy item. Specific postures can lead to exponential increases in intradiscal pressure, which can predispose to disc injury.18 Exposure to vibrational energy combined with sustained lateral flexion and rotation may also predispose to herniation.16 The exact history of the incident and the presence of preexistent back or leg pain must be explored; this is particularly important in work-related injuries.
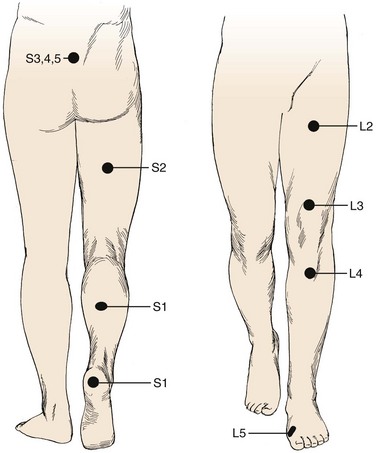
Pain is the most common complaint. Axial back pain is typically present, although some patients do not have this complaint. Radicular pain is more typical and often the more “treatable” of the complaints. The pattern of lower extremity radiation depends on the level of the herniation. Lower lumbar or lumbosacral disc herniations can lead to the classic symptoms of pain radiating below the knee. Often pain extends into the foot and can follow a dermatomal distribution. S1 radicular pain may radiate to the back of the calf or the lateral aspect or sole of the foot. L5 radicular pain can lead to symptoms on the dorsum of the foot (Fig. 46–7). Radiculopathy from involvement of the upper lumbar roots can lead to more proximal symptoms. L2 and L3 radiculopathy can produce anterior or medial thigh and groin pain. Groin pain may also be indicative of L1 pathology. Radicular pain can be difficult to discern and is often not “classic.” Many patients do not exhibit pain in a specific dermatomal distribution, or the radiation does not extend along the entire leg. It may radiate only into the hip region or just the foot or any portion of the leg.
The influence of social and psychological factors on the outcomes of disc surgery has been well documented. It is highly recommended to obtain a social and at least cursory psychiatric history. Prescription use of antidepressants is an important clue, although depression is often undiagnosed and untreated at the time of initial presentation. Other personality factors, such as chronic headaches, hysteria, hypochondriasis, nervous disorders, and impulsivity, can also be influential.13,35 Work history, pending litigation, and type of work should be obtained. A history of smoking is an independent risk factor for low back pain and a risk factor for a poor result after back surgery.35,36
Physical Examination
Inspection
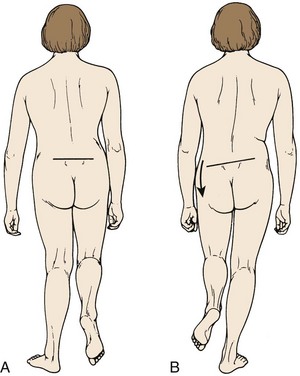
Inspection is the first step in the physical examination. As the patient walks into the examining room, gait should be observed. A sciatic list may be present, usually manifested as the patient leaning away from the side of leg pain. This sciatic list is thought to be associated with a paracentral herniation lateral to the nerve root. Axillary herniations may cause a list toward the side of herniation. The list is an attempt to relieve neuromeningeal tension by drawing the nerve root away from the herniated fragment. Another feature of gait that should be noted is a wide-based gait, indicative of lumbar or more cranial canal stenosis. A footdrop or foot slapping gait may occur with L4 or L5 paresis. A Trendelenburg gait can suggest hip abductor weakness (Fig. 46–8), which may be a clue to L5 nerve root compression because the gluteus medius is most often an L5 dominant muscle.
Neurologic Examination
A neurologic examination is required in all patients with suspected herniated discs. Sensation of light touch is tested along dermatomes from L1 to S1. Standard dermatomal charts can be helpful, but there is variability among individuals, and so this is highly subjective. In testing the upper lumbar roots, there is often a significant amount of overlap. The most discrete levels of testing are for L4, L5, and S1 nerve roots.37 These nerve roots are the most often affected by lumbar disc herniations (Table 46–1). L4 sensory function is tested at the medial ankle; L5, at the first webspace between the great and second toes; and S1, at the lateral aspect of the sole of the foot. Sensation is difficult to “grade.” It is more useful to document sensation as normal, diminished, or absent. Sensory function should be compared with the contralateral side because this may help detect differences. The examiner should be wary of the presence of a glove-and-stocking distribution sensory loss, which can indicate a peripheral neuropathy, such as associated with diabetes, or functional overlay—as it is not anatomic.
TABLE 46–1 Prevalence of Back Pain and Sciatica in Adults
| Characteristic | Prevalence (%) |
|---|---|
| Any low back pain | 60-80 |
| Any low back pain persisting at least 2 wk | 14 |
| Low back pain persisting at least 2 wk at a given time (point prevalence) | 7 |
| Back pain with features of sciatica lasting at least 2 wk | 1.6 |
| Lumbar spine surgery | 1-2 |
From Deyo RA, Loeser J, Bigos S: Herniated lumbar intervertebral disc. Ann Intern Med 112:598-603, 1990.
The motor examination should proceed in a routine manner. In the lower extremity, it is better to test movements rather than specific muscles. S1 motor function is assessed by testing plantar flexion, whereas L5 is tested by toe dorsiflexion, particularly the great toe (extensor hallucis longus), and hip abduction. L4 involvement most often affects ankle dorsiflexion (anterior tibialis), although quadriceps function can be compromised. There is a significant amount of overlap of upper lumbar motor innervation. Knee extension can be considered L3 function (although L2 and L4 contribute); hip flexion, an assessment of L1-2 function; and hip adduction, an assessment of L2 function. Motor function is graded as 0 to 5, with 5 being full strength against active resistance (Table 46–2). In particular, S1 function should be assessed by asking the patient to toe raise repeatedly or toe-walk. Because of the enormous strength of the gastrocnemius complex, even a weakened muscle can overcome the examiner’s hand. Toe-walking can show smaller differences, however, from side to side by using the weight of the patient’s body as the resistance. Repetitive toe raising may help detect smaller differences.
TABLE 46–2 Motor Strength Grading System by Physical Examination
| Grade | Definition |
|---|---|
| 0 | No visible muscle contraction at all |
| 1 | Visible muscle contraction; no joint movement |
| 2 | Can move joint but not overcome gravity |
| 3 | Able to overcome gravity but cannot overcome any examiner resistance |
| 4 | Able to overcome some, but not full, examiner resistance |
| 5 | Full strength; able to resist full examiner force |
Specific Tests
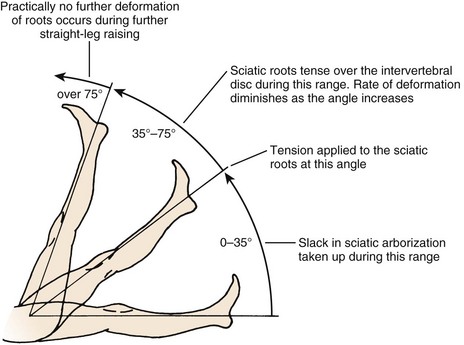
The straight-leg raise (SLR) test is an extremely useful provocative test in examining patients with a herniated disc (Fig. 46–9). The classic test is performed with the patient in the supine position. The heel of a relaxed leg is cupped by the examiner’s hand and elevated slowly. The knee is kept in extension while the hip is flexed. The test is considered positive if sciatic pain is reproduced between 35 degrees and 70 degrees of elevation. Studies have determined that in the first 35 degrees of elevation, the slack in the nerves is taken up, and at 35 degrees or more, tension is placed on the nerves. More than 70 degrees of elevation causes no further stretch of the nerve roots. The SLR test is best for eliciting L4, L5, or S1 radiculopathy. It is not useful for upper lumbar roots, for which a femoral stretch test should be used. A positive SLR test is indicative of nerve root compression in 90% of cases.38 It does not implicate a herniated disc as the source of compression, however, because foraminal encroachment or other mass lesions can lead to a positive SLR test as well.
The so-called slump test is a variant of the Lasègue test and the SLR test. This test is performed in the seated position; the patient is asked to flex the thoracic and lumbar spine while fully flexing the neck. Next, the SLR test is performed while the foot is dorsiflexed on the same side, as denoted by the Lasègue test. The combination of these maneuvers adds cephalad gliding of the spinal cord to the examination, whereas the SLR test and Lasègue test by themselves produce only caudal tension on the nerve roots. A more recent study found the slump test was more sensitive than the SLR test in patients with lumbar disc herniations, whereas the SLR test was more specific.39
Differential Diagnosis
Diagnostic Imaging
Magnetic Resonance Imaging
MRI is the most popular modality for advanced imaging of lumbar disc herniations. MRI is superior to CT in delineating soft tissues. The disc and fragments that may have herniated from it are readily visualized. Free fragments (sequestered) can be differentiated from extruded disc herniations (Fig. 46–10), and a symmetrical bulge can be differentiated from a contained protrusion. The neural elements themselves are well visualized. Neural encroachment can be detected within the spinal canal, the foramina, or extraforaminally. MRI is also useful in differentiating disc herniations from tumors, vascular anomalies, or bony compression.
Numerous features of a herniated disc can be noted on MRI. The size and type of disc herniation can be reliably determined using MRI, which may have prognostic significance.11,40,41 Carragee and Kim11 correlated outcomes with herniated fragment size and its effect on canal area. Larger discs (>6 mm) were more likely to have a positive SLR test or femoral stretch test (Wasserman sign). In the operative group, larger discs were predictive of a better outcome. The fair and poor outcomes in operative patients were in patients with small discs (<6 mm).
Attempts to correlate MRI findings with clinical symptoms have been made. In 33 patients in whom disc herniation was diagnosed clinically and 5 control patients with low back pain alone, Kikkawa and colleagues42 performed three-dimensional MRI using a fast low angle shot (FLASH) with gadolinium enhancement. Dorsal root ganglion enhancement was found to be nonspecific, occurring in controls and sciatica patients. Enhancement of the root proper was detected, however, in 11 of 30 symptomatic patients, with patients having a statistical tendency for more severe motor involvement. There was no significant association of diffuse versus local enhancement with the positivity of the SLR test or sensory changes. Central compression of the cauda equina did not lead to enhancement in any cases. Although these results are modest, they suggest a future use of gadolinium-enhanced MRI as a noninvasive method of determining the microvascular response to compression of neural structures.
Komori and colleagues41 studied the significance of enhancement around the herniated fragment itself. Patients with radiculopathy underwent initial and follow-up gadolinium-enhanced MRI to correlate clinical improvement with the degree of enhancement. Patients with marked decrease in size of the herniation showed good clinical resolution. This resolution was most significant in the “migrating”-type discs, which were closest to sequestered discs according to the authors’ description. Decrease in fragment size was associated with a gradual increase in the area of enhancement in 17 of 22 sequestered disc herniations, all of which had improvement of radicular pain. Five cases of sequestered discs without enhancement or size decrease had a poor clinical result. Enhancement was less marked in extruded versus sequestered herniations; however, herniations that did show enhancement had a significantly better clinical course. From these data, Komori and colleagues41 recommended this test as a prognostic tool in guiding the treatment of patients with extruded or sequestered herniated discs.
Of more recent interest is the influence of posture on the MRI appearance of discs and their relationship to the neural structures. Because images are traditionally acquired in the supine position, the spine is not axially loaded as it is during everyday activities. Weishaupt and colleagues43 performed positional MRI in patients with low back or leg pain for 6 weeks that was not responding to conservative treatment. Images were obtained in the usual supine position and with a seated flexed and extended posture. Changes in foraminal size and neural compression occurred with flexion and extension. Changes in foraminal size correlated with increased pain scores. These findings are probably most significant for low-grade herniations (i.e., bulges or protrusions) in which there is still a fixed-volume system within the disc space provided by an intact outer annular layer. Similar findings have been shown using dynamic functional plain myelography.44
Magnetic Resonance Imaging in the Postoperative Spine
As the modality of choice for imaging the neural structures, MRI is frequently obtained. Because of edema, hematoma, and formation of surgical scar, MRI is best delayed until 6 months after surgery,45 if symptoms allow. The main challenge is differentiating scar from new-onset disc. On standard T1-weighted sequences, this differentiation can be difficult. In the early days of MRI, T2-weighted images were not as useful because of longer scan times with inadequate magnet strength.46
Evidence suggests that sophisticated T2 image analysis might supplant the need for gadolinium-enhanced MRI. Barrera and colleagues47 compared different imaging sequences with and without gadolinium contrast agent. These investigators documented 100% sensitivity for detecting scar for T2-weighted turbo-spin echo (TSE) and fluid attenuated inversion recovery (FLAIR) sequences compared with T1-weighted images with gadolinium. Specificity was 94% and 92% for TSE T2 and FLAIR images. Barrera and colleagues47 concluded that standard TSE T2 images acquired using a rapid sequence are extremely sensitive and specific in distinguishing disc from scar in most cases and that the use of gadolinium contrast agent should be reserved for the rare situation in which that distinction cannot be made. These recommendations are supported by others.46
Grane and Lindqvist45 studied the role of gadolinium enhancement of the nerve roots after discectomy. These investigators found intradural (within the cauda equina) nerve root enhancement in 59% of patients with recurrent clinical symptoms. Recurrent symptoms occurred, however, in 84% of patients with focal (extradural, after the nerve root has existed the cauda equina) enhancement and 86% of patients with nerve root thickening. Enhancement occurred in patients with and without evidence of nerve root displacement by scar or disc. This finding indicates that although symptoms may correlate with MRI enhancement, it is not associated with a compressive mass lesion.
In an early report on the use of MRI without gadolinium, Bundschuh and colleagues48 studied 20 patients after failed disc surgery who had a strong likelihood of undergoing further surgery. In 14 patients, CT with contrast agent was also performed. The authors found that free fragments of disc had a mildly increased signal on T1 images compared with scar, whereas scar and disc were similarly hyperintense on T2 images. Overall, Bundschuh and colleagues48 believed that MRI was at least comparable to CT with contrast agent in differentiating scar from disc, confirmed by intraoperative findings.
Myelography
Plain myelography previously was the imaging modality of choice in detecting herniated discs. It involves injection of intrathecal contrast material to outline the boundaries of the subarachnoid space and silhouette the enclosed neural elements. It is invasive and cannot show compression beyond the confines of the subarachnoid space. Extradural compression caused by a foraminal or extraforaminal disc can be missed. Advantages of myelography are that it is a dynamic test because images can be made with the patient standing.44 Myelography should be reserved for cases in which noninvasive imaging, such as CT or MRI, are nondiagnostic, equivocal, or contraindicated. Currently, myelography is rarely used for the routine workup of herniated discs. When used, it is usually followed by a CT scan.
Computed Tomography
Some disc herniations can contain gas (Knuttson phenomena), noted on CT images. Mortensen and colleagues49 reported four such cases that responded well to surgical discectomy. It is unknown if the gas forms before or after herniation. The clinical significance of the gas is not well understood. Ford and colleagues50 determined that intradiscal gas is composed predominantly of nitrogen.
Natural History
In a widely quoted retrospective study, Saal and Saal5 found a 90% good or excellent outcome in patients treated nonoperatively for a lumbar disc herniation diagnosed by clinical examination and CT. Inclusion criteria were strict, including patients with SLR test positive at 60 degrees or less, leg pain greater than back pain, and electromyographic evidence of radiculopathy. Of patients, 92% returned to work. Nonoperative treatment consisted of aggressive physical therapy and back school education. A possible confounding factor is that many patients were referred for a second opinion regarding surgical versus nonsurgical treatment because they were anxious to avoid surgery. This may have introduced preselection bias error because the authors of the study were not surgeons. Concern has been raised about eventual fibrosis formation with nonoperative treatment of herniated discs. In a follow-up MRI study,6 the same investigators documented no increased risk for perineural fibrosis or adhesions with nonsurgical management.
Other authors have reported more modest results. In the nonoperative arm of Weber’s10 classic randomized study, the long-term outcome of lumbar disc herniations was observed in 49 patients. Inclusion criteria were clinical signs and symptoms of L5 or S1 radiculopathy in addition to myelographic evidence of nerve root compression. Treatment included full-time bed rest for 1 week followed by partial bed rest the 2nd week and back school instruction as an inpatient. At 1 year, 33% had good results, 49% had a fair result, and 18% had a poor result. At 4 years, good results were reported in 51%, fair results were reported in 39%, and poor or bad results were reported in 10%. Because the tiered system is slightly different than that used by Saal and Saal,5 a direct comparison of the studies is difficult. If Weber’s good and fair results are equated to Saal’s excellent and good results, an 89% success rate achieved in the former at 4 years may be considered comparable to the latter’s 90% success. Many of Saal and Saal’s patients ultimately dropped out of the study and underwent surgical discectomy so that their 90% success rate might represent an overestimation.
In another nonoperative arm of a comparative study, 10-year follow-up results from the prospective Maine Lumbar Spine Study showed 61% improvement in the predominant symptom, 40% resolution of low back symptoms, and 56% satisfaction rate.7 Work and disability status were comparable between operative and nonoperative groups in this investigation. Similar findings were reported for the observational cohort of the Spine Patient Outcomes Research Trial (SPORT).8
Methods of Nonoperative Treatment
Physiotherapy
Bed rest should be limited to no more than 2 to 3 days.51 Greater periods of inactivity can potentiate prolonged disability and continued or augmented pain. Exercise therapy and physical rehabilitation should be included in the nonoperative care of herniated discs. Treatment goals are to restore strength, flexibility, and function that were lost secondary to pain, splinting, and spasm. Postural education to avoid activities that can increase intradiscal pressure or neuromeningeal tension or both should be provided.
Adjunctive modalities can aid in relieving some associated symptoms. These modalities include ultrasound treatment, electrical stimulation, and massage. These may be helpful in short-term, symptomatic relief of back pain. Traction is also commonly prescribed. It theoretically may diminish intradiscal pressure, increase foraminal dimensions, and possibly relieve radicular pain secondary to herniated discs.52 The role of chiropractic manipulation is controversial. Although some patients believe that manipulations have “reversed” the herniation, there is no evidence to support the ability of chiropractic manipulation to alter the normal or pathologic morphology of the disc.53
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree