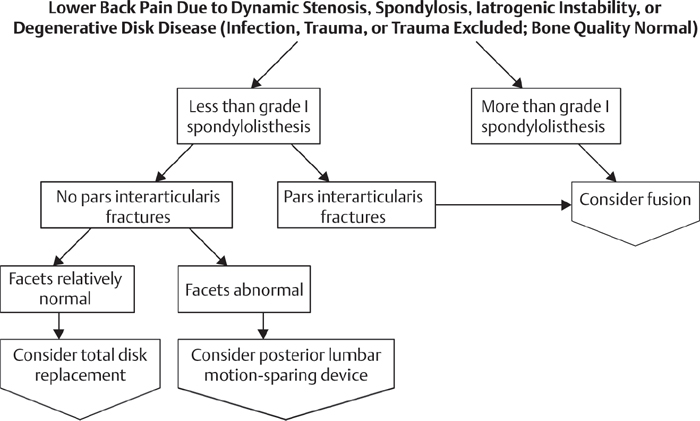
63 Chronic lower back pain (CLBP) is an ailment of high prevalence in the United States with major socioeconomic impact among working-age individuals. The underlying cause of CLBP is multifactorial, and many potential anatomic regions are potential sources for pain. The two main etiologies of low back pain are believed to be degenerative disk disease (DDD), in 10–39% of the cases, and facet arthropathy.1,2 Disk desiccation, loss of height, and fissures/tears of the annulus fibrosus have all been implicated as factors contributing to altered biomechanics of the spinal functional unit, leading to pathologic micromotion. This micro-motion is believed to result in instability and to generate pain.1,2 Nonoperative therapies are the first line of treatment for discogenic CLBP, with the majority of patients responding favorably and few requiring more aggressive treatments. In refractory cases, surgical management may be indicated. The current “gold standard” is lumbar arthrodesis. While there is controversy regarding the role of fusion in management of degenerative back pain, there are studies that have demonstrated superior results compared with nonoperative cohorts. Patients receiving arthrodesis have been reported to have better Oswestry Disability Index (ODI) scores, patient satisfaction scores, and return-to-work rates than their nonoperative counterparts.3 Fusion of a given spinal level is believed to alter the biomechanics of the adjacent motion segments: the creation of a rigid segment increases the stress at the adjacent levels. This increased stress may lead to an increased risk of developing degenerative disk disease at the adjacent levels. Initially described in the 1950s, total disk replacement (TDR) of the lumbar spine was developed as an alternative to arthodesis.4 The concept of lumbar arthroplasty involves reproducing the motion segment with preservation of the sagittal balance and maintaining the normal physiological load across the functional spinal unit. This is hypothesized to result in less stress across the adjacent segments and to lower the risk of developing adjacent disk disease in the future relative to a fusion procedure.5 Many different TDR prostheses have been created, with varying degrees of constraint. The motion across the intervertebral disk space can be classified with six degrees of freedom, with rotation and translation occurring in all three axes. The L5–S1 disk has the highest level of freedom of motion, averaging 19.6 degrees. With rostral progression, the freedom of motion decreases, with L1–L2 averaging 12.7 degrees.6,7 The initial attempts to replicate this motion were reported in the 1960s, when Fernstrom implanted stainless steel spheres into the disk space. This implant fell out of favor because of reported high failure rates. In the 1980s the Stefee disk was introduced, consisting of metal/plastic plates with rubber interfaces. This prosthesis would eventually be discontinued secondary to catastrophic failure of the rubber core.8 Modern TDR constructs rely on either a metal on ultra-high-molecular-weight polyethylene (UHMWPE) interface or a metal-on-metal interface. Both the Charite TDR (Depuy Spine, Raynham, MA) and Pro-Disc-L TDR (Synthes Spine, West Chester, PA) have been approved for clinical use in the United States. Both consist of two chrome-cobalt endplates interfacing with a UHMWPE core. While the Charite TDR is approved only for single-level arthroplasty (L4–L5 or L5–S1), the Pro-Disc-L is approved for one- to two-level replacements from L3 to S1. The Maverick (Medtronic, Memphis, TN) and Flexicore TDR are two metal-on-metal constructs; the Maverick has not entered the U.S. market because of ongoing patent litigation. Patient selection for TDR is paramount, as the current indications for the TDR preclude the majority of fusion candidates. It has been estimated that anywhere from 0% to 25% of all fusion candidates meet the criteria for a TDR.5,9 According to Bertagnoli and Kumar, the ideal candidate for a TDR is a patient with refractory low back pain of discogenic origin at a single disk level with the involved disk having a height of 4 mm or greater.10 The patient should also have no neurologic deficits and have an intact posterior column. The use of TDR should be avoided in patients presenting with advanced facet arthrosis, degenerative scoliosis, lytic spondylolithesis, and spondylolithesis that is greater than Grade 1. Other contraindications include active systemic/local infection, poor bone stock, spinal stenosis from bony components, and component spatial incompatibility. Furthermore, a TDR will result in an increased sacrovertebral angle; this phenomenon does not occur when a lordotic interbody construct is placed in an anterior lumbar interbody fusion (ALIF). All patients who may be considered a candidate for a TDR should undergo a thorough neurologic examination. Inspection begins with gait analysis, checking for any gait disturbance, waddling, or antalgic gait. The skin overlying the spine should be inspected for any evidence of dimpling. Sensation should be inspected along the lumbar/sacral dermatomes, followed by testing motor function of at least one muscle group per myotome. Deep tendon reflexes, clonus, and the presence of tension signs should all be tested. The initial workup should include anteroposterior (AP) and lateral radiographs to evaluate for any bony abnormalities. Radiographs will give details regarding scoliosis, spondylolisthesis, lysis, facet arthrosis, pars defects, spina bifida occulta, osteophyte formation, and calcification of the disk. Magnetic resonance imaging (MRI) is excellent in evaluation of the facet joint capsule and the level of disk degeneration. A computed tomography (CT) scan will give greater detail regarding the bony anatomy if needed after review of plain radiographs. In patients over the age of 50, consideration should be given to obtaining a dual-energy X-ray absorptiometry (DEXA) scan to evaluate for osteoporosis. The role of diskogram/diskoblock is controversial, although many surgeons require a concordant diskogram at the level of a degenerated disk as a prerequisite for surgical intervention. The surgical approach for placing a TDR is via the anterior trans/retroperitoneal approach. Both approaches have acceptable safety profiles; however, the retroperitoneal approach has been reported to be associated with a lower incidence of retrograde ejection, peritoneal adhesions, and postoperative ileus compared with the anterior approach.11 Depending on the construct design and insertion method, implanting a TDR may require a larger incision and greater mobilization of the vessels than a comparable ALIF procedure. Insertion of the construct is often guided with AP and lateral fluoroscopy to ensure correct placement and seating of the implant. The implant should be placed flush with the posterior edge of the vertebral body to emulate the physiologic center of rotation of the lumbar segment most closely.12 Currently, there are 16 prospective comparative cohort studies and two randomized control trials involving the safety and efficacy of lumbar TDR versus lumbar arthrodesis.13 In 2005, Blumenthal et al. reported the 2-year results of the FDA investigational device exemption (IDE) trial of the Charite TDR. This was a randomized controlled trial with greater than 90% follow-up.14 They found that the TDR group reported greater satisfaction at 2 years and better visual analog scale (VAS) and ODI scores at 1 year compared with the fusion cohort. The TDR group also had a shorter hospital stay and faster recovery relative to the fusion group. Complications were similar, with no significant differences. Similarly, in 2007 Zigler et al. reported the 2-year data of the FDA IDE trial involving the ProDisc-L.15 At 2 years the ProDisc-L cohort had higher ODI and VAS scores compared with the fusion group. The arthroplasty group also had a shorter operative time, hospital stay, and lower estimated blood loss (EBL).15 Guyer et al. in 2009 published the 5-year results of the Charite TDR FDA IDE trial.16 Only 44% of the original cohort patients were included. The overall success at 5 years was 58% for the Charite group and 51% for the fusion group; this was statistically significant. The ODI, VAS pain scores, and Short Form 36 (SF-36) scores were comparable between both groups; however, the TDR group had a substantially higher return-to-work rate compared with the fusion group (65.6% versus 46.5%). Lamaire et al. in 2005 reported minimum 10-year follow-up results in 100 patients treated with Charite TDR.17 He reported a greater than 90% return-to-work rate and 10% poor result rate. Lumbar TDR complications can be categorized as related to the surgical approach, prosthesis, infection, or miscellaneous. Surgical approach–related complications include injury to the great vessels, nerve root injury, and retrograde ejaculation. Prosthesis-related complications include implant subsidence, end-plate fracture, and dislocation of the prosthesis. Prosthesis dislocation is a catastrophic complication, with a reported prevalence ranging from 0% to 25%, with the risk of dislocation substantially increasing with multilevel surgery.18–21 As with most anterior spinal surgeries, the infection risk is low; however, other complications, such as heterotopic ossification (HO), spontaneous fusion, prosthesis loosening, facet arthrosis, and osteolysis, have up to a 20% prevalence among patients.22 In some series the prevalence of HO can be as high as 30%.23 Interestingly, the presence of HO has not been shown to affect the overall clinical outcome: the ODI scores among patients with HO were no different from those without.23 Revision of a TDR is a daunting task. The most feared major complication associated with revision surgery is injury to the great vessels that can occur while dissecting through scar tissue or while mobilizing the great vessels with retractors. The incidence of great vessel damage during revision surgeries has been reported to approach 17%.24 Although modern lumbar TDR constructs have limited long-term outcome data, the short-term data are promising. In select patients with CLBP and DDD, TDR may provide an alternative to fusion. In the two large FDA IDE TDR head-to-head inferiority studies, the TDR group reported slightly better patient satisfaction scores and higher return-to-work rates versus arthrodesis.25 Objectively, TDR provides an operation that may result in lower blood loss, shorter intraoperative times, and shorter hospital stays compared with anterior arthrodesis.26 With the strict and narrow indications for TDR, only a handful of patients with lumbar DDD will be candidates. However, in this small subset patient population, a TDR represents a viable alternative to interbody fusion. Long-term outcomes will eventually shed light on the incidence of adjacent-segment disease with TDR versus fusion. 1. Fischgrund JS, Montgomery DM. Diagnosis and treatment of discogenic low back pain. Orthop Rev 1993;22(3):311–318 PubMed 2. Schwarzer AC, Aprill CN, Derby R, Fortin J, Kine G, Bogduk N. The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine 1995;20(17):1878–1883 PubMed 3. Fritzell B. Detection of adverse events: what are the current sensitivity limits during clinical development? Vaccine 2001;20(Suppl 1):S47–S48 PubMed 4. Fernström U. Arthroplasty with intercorporal endoprothesis in herniated disc and in painful disc. Acta Chir Scand Suppl 1966;357:154–159 PubMed 5. Wong DA, Annesser B, Birney T, et al. Incidence of contraindications to total disc arthroplasty: a retrospective review of 100 consecutive fusion patients with a specific analysis of facet arthrosis. Spine J 2007;7(1):5–11 PubMed 6. Yoshioka T, Tsuji H, Hirano N, Sainoh S. Motion characteristic of the normal lumbar spine in young adults: instantaneous axis of rotation and vertebral center motion analyses. J Spinal Disord 1990;3(2):103–113 PubMed 7. Pearcy MJ, Bogduk N. Instantaneous axes of rotation of the lumbar intervertebral joints. Spine 1988;13(9):1033–1041 PubMed 8. Enker P, Steffee A, Mcmillin C, Keppler L, Biscup R, Miller S. Artificial disc replacement. Preliminary report with a 3-year minimum follow-up. Spine 1993;18(8):1061–1070 PubMed 9. Huang RC, Lim MR, Girardi FP, Cammisa FP Jr. The prevalence of contraindications to total disc replacement in a cohort of lumbar surgical patients. Spine 2004;29(22):2538–2541 PubMed 10. Bertagnoli R, Kumar S. Indications for full prosthetic disc arthroplasty: a correlation of clinical outcome against a variety of indications. Eur Spine J 2002;11(Suppl 2):S131–S136 PubMed 11. Bendo JA, Quirno M, Errico T, Spivak JM, Goldstein J. A comparison of two retroperitoneal surgical approaches for total disc arthroplasty of the lumbar spine. Spine 2008;33(2): 205–209 PubMed 12. Dooris AP, Goel VK, Grosland NM, Gilbertson LG, Wilder DG. Load-sharing between anterior and posterior elements in a lumbar motion segment implanted with an artificial disc. Spine 2001;26(6):E122–E129 PubMed 13. van den Eerenbeemt KD, Ostelo RW, van Royen BJ, Peul WC, van Tulder MW. Total disc replacement surgery for symptomatic degenerative lumbar disc disease: a systematic review of the literature. Eur Spine J 2010;19(8):1262–1280 PubMed 14. Blumenthal S, McAfee PC, Guyer RD, et al. A prospective, randomized, multicenter Food and Drug Administration investigational device exemptions study of lumbar total disc replacement with the Charite artificial disc versus lumbar fusion: part I: evaluation of clinical outcomes. Spine 2005;30(14):1565–1575, discussion E387–E391 PubMed 15. Zigler J, Delamarter R, Spivak JM, et al. Results of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine 2007;32(11):1155–1162, discussion 1163 PubMed 16. Guyer RD, McAfee PC, Banco RJ, et al. Prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of lumbar total disc replacement with the Charite artificial disc versus lumbar fusion: five-year follow-up. Spine J 2009; 9(5):374–386 PubMed 17. Lemaire JP, Carrier H, Sariali H, Skalli W, Lavaste F. Clinical and radiological outcomes with the Charité artificial disc: a 10-year minimum follow-up. J Spinal Disord Tech 2005;18(4): 353–359 PubMed 18. Guyer RD, Geisler FH, Blumenthal SL, McAfee PC, Mullin BB. Effect of age on clinical and radiographic outcomes and adverse events following 1-level lumbar arthroplasty after a minimum 2-year follow-up. J Neurosurg Spine 2008;8(2):101–107 PubMed 19. Di Silvestre M, Bakaloudis G, Lolli F, Vommaro F, Parisini P. Two-level total lumbar disc replacement. Eur Spine J 2009;18(Suppl 1):64–70 PubMed 20. Geisler FH, Guyer RD, Blumenthal SL, et al. Patient selection for lumbar arthroplasty and arthrodesis: the effect of revision surgery in a controlled, multicenter, randomized study. J Neurosurg Spine 2008;8(1):13–16 PubMed 21. Geisler FH, Guyer RD, Blumenthal SL, et al. Effect of previous surgery on clinical outcome following 1-level lumbar arthroplasty. J Neurosurg Spine 2008;8(2):108–114 PubMed 22. Park CK, Ryu KS, Jee WH. Degenerative changes of discs and facet joints in lumbar total disc replacement using ProDisc II: minimum two-year follow-up. Spine 2008;33(16): 1755–1761 PubMed 23. Park SJ, Kang KJ, Shin SK, Chung SS, Lee CS. Heterotopic ossification following lumbar total disc replacement. Int Orthop 2011;35(8):1197–1201 PubMed 24. McAfee PC, Geisler FH, Saiedy SS, et al. Revisability of the Charite artificial disc replacement: analysis of 688 patients enrolled in the U.S. IDE study of the Charite Artificial Disc. Spine 2006;31(11):1217–1226 PubMed 25. Guyer RD, McAfee PC, Banco RJ, et al. Prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of lumbar total disc replacement with the Charite artificial disc versus lumbar fusion: five-year follow-up. Spine J 2009;9(5): 374–386 PubMed 26. Zigler J, Delamarter R, Spivak JM, et al. Results of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine 2007;32(11):1155–1162, discussion 1163 PubMed Guyer RD, McAfee PC, Banco RJ, et al. Prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of lumbar total disc replacement with the Charite artificial disc versus lumbar fusion: five-year follow-up. Spine J 2009;9(5):374–386 PubMed This is a 5-year follow-up to comparison study of the Charite artificial disk replacement in a head-to-head noninferior study versus traditional fusion. Analysis shows that Charite has higher, but not significant, results versus fusion in return-to-work rates, ODI, and complications. Charite is determined to be a noninferior product. Enker P, Steffee A, Mcmillin C, Keppler L, Biscup R, Miller S. Artificial disc replacement. Preliminary report with a 3-year minimum follow-up. Spine 1993;18(8):1061–1070 PubMed This is an analysis of 3-year data on the earlier constructs of the modern TDR. Analysis points to potent pitfalls, which replacement constructs were designed to overcome, from fusion. Initial data prove viability but not sustainability with the examined devices. Huang RC, Lim MR, Girardi FP, Cammisa FP Jr. The prevalence of contraindications to total disc replacement in a cohort of lumbar surgical patients. Spine 2004;29(22):2538–2541 PubMed This article provides an in-depth look at the selection criteria of the TDR surgery. The study yields an estimate that only 5% of all fusion candidates do not have a single contraindication to replacement surgery. Park CK, Ryu KS, Jee WH. Degenerative changes of discs and facet joints in lumbar total disc replacement using ProDisc II: minimum two-year follow-up. Spine 2008;33(16):1755–1761 PubMed This study examines the long-term effects of the ProDisc II construct on adjacent levels and supporting structures. It compares results of the ProDisc II with long-term effects of the Charite construct. It focuses on adjacent-level disease progression and facet changes.
Intervertebral Disk Arthroplasty of the Lumbar Spine
![]() Workup
Workup
Physical Examination
Spinal Imaging
Operative
![]() Outcome
Outcome
![]() Complications
Complications
References
Suggested Readings
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







