Chapter 50 Immunosuppressive Drug Therapy
Alkylating Agents
Of the more than 12 alkylating agents that are currently in use, CyX, chlorambucil, and mechlorethamine (nitrogen mustard) have been most widely used to treat patients with SLE. The earliest use of alkylating agents, reported by Osborne and associates1 in 1947, was the topical application of nitrogen mustard in cutaneous lupus, followed in 1949 with the description by Chasis2 of rapid and dramatic responses to nitrogen mustard in LN—patients with nephrotic syndrome were sometimes observed to begin diuresing within 1 day of treatment. Mechlorethamine has since been largely abandoned because of toxicity, although it is arguable that those patients with the worst of symptoms might yet benefit even now from such aggressive therapy during initiation of long-term treatment with a better-tolerated compound such as MMF.
Cyclophosphamide
Active metabolites of CyX include 4-hydroxycyclophosphamide, aldophosphamide, phosphoramide mustard, and acrolein, all of which have differing rates of synthesis, half-lives, immunologic effects, and toxicities.3 Serum levels of these metabolites are not routinely measured; hence, dose adjustment in patients with renal or hepatic failure is largely empiric. Doses should be reduced approximately 30% in patients with a creatinine clearance of less than 30 mL/min. Some investigators have proposed stepwise reduction as renal function declines.4 Furthermore, CyX is incompletely cleared by dialysis; therefore the dose should be lowered for dialysis patients as well. The effect of hepatic insufficiency on CyX toxicity is incompletely understood, in part because the liver is responsible for both the production of active metabolites and their degradation. CyX is metabolized not only in the liver but also in lymphocytes and transitional epithelial cells in the bladder, which may result in local toxicity or immunosuppression or both. CyX may have toxic and/or therapeutic effects in cells that are not actively dividing, as well as in dividing cells.
CyX produces dose-related lymphopenia. IVC reduces the population of cluster of differentiation 4 (CD4+) and cluster of differentiation 8 (CD8+) lymphocytes and B cells, with a more significant reduction of CD4+ lymphocytes and B cells during monthly therapy.5–7 After the cessation of monthly therapy, B-cell populations rapidly return to baseline, but CD4+ populations remain relatively suppressed during less intensive IVC therapy, resulting in prolonged reduction of the CD4+/CD8+ ratio.6
Persistent reduction of the number of cluster of differentiation 19 (CD19+) lymphocytes 6 months after the completion of therapy has been reported,8 and specific reduction of B-cell function has been described.9 Reduction of autoantibody production has been demonstrated in patients with SLE who are treated with both oral CyX and IVC and in patients with rheumatoid arthritis (RA) who are treated with oral CyX. Despite the reduction of pathogenic autoantibody production, reduction of overall levels of immunoglobulin (Ig) G, IgA, and IgM, and IgG subclasses has not been observed in the authors’ patient population. This suggests that specific suppression of autoantibody production is a function of CyX when used in therapeutic doses and may underlie its beneficial action in patients with SLE.
Daily oral CyX, which has been used for induction in some recent nephritis trials, 10,11 is usually initiated at 1 to 2 mg/kg/day. The use of a standard maximum dosage of 2 mg/kg/day, with dose reduction in the presence of leukopenia (white blood cell [WBC] count of less than 3500 cells/mm3) or neutropenia (WBC count of less than 1000 cells/mm3), is a common practice. Gradually increasing the dose of CyX with the goal of producing mild leukopenia is another treatment strategy. Although these approaches have not been directly compared in a single trial, it is likely that the avoidance of leukopenia, coupled with prophylaxis against Pneumocystis carinii, may significantly reduce morbidity and mortality from infection during daily CyX therapy. Monitoring for toxicity includes weekly complete blood counts (CBCs) initially advancing to monthly when stable, urinalyses to detect hemorrhagic cystitis, and annual urine cytologic studies.
Hemorrhagic Cystitis and Carcinomas of the Bladder
Acrolein, which is directly toxic to the bladder, can cause hemorrhagic cystitis, a premalignant lesion identified in 50% of patients receiving CyX who eventually develop transitional cell carcinoma of the urinary tract; and bladder fibrosis, which has been reported in 5% to 34% of patients receiving daily oral CyX. Hemorrhagic cystitis, which may include either microscopic or gross hematuria (and may be life threatening), mandates permanent discontinuation of CyX, and lifetime annual urologic follow-up. The risk of bladder carcinoma associated with daily CyX therapy is dose dependent and is significantly increased after a total dose of 30 g. In a study of patients with RA, 9 of the 119 patients treated with CyX developed bladder carcinomas after 20 years; of these, 7 received more than 80 g of CyX.12–15
Other Malignancies
Development of malignancies after CyX administration is well described in patients with rheumatic diseases, particularly RA and granulomatosis with polyangiitis (Wegener granulomatosis). Non–urinary tract neoplastic complications of CyX include skin cancers and hematologic malignancies, as well as cervical atypia, which can be observed even in patients who have received cumulative doses of CyX of less than 10 g. In those patients who have received 80 to 120 g cumulative CyX doses, myelodysplastic syndromes are observed, characterized by monozomy-5 or monozomy-7 or both. Long-term follow-up studies by Baltus and associates13 and Baker and colleagues14 of patients with RA and treated with oral CyX have established approximately 10% additional incidence of malignancy, compared with age-matched controls after a total dose of 30 g. Doses of less than 10 g are almost certainly safer; doses of 100 g or more are even more likely to produce malignancies. Radis and others15 reported a 20-year follow-up of the original study by Baker and colleagues14 and showed continued occurrence of CyX-induced malignancies; after 20 years, only 40% of the original patient population remained free of cancer.
IVC therapy of patients with lupus has not yet been associated with a statistically significant increase in solid tumors, probably because of the lower cumulative doses and the use of intravenous hydration to protect the urinary tract, although a significant increase in cervical intraepithelial neoplasia exists.16
Pulmonary Toxicity
Pulmonary toxicity is an infrequent complication during therapy with CyX. Acute interstitial pneumonitis is the most frequently encountered pulmonary involvement of CyX therapy. Pulmonary injury as a result of CyX therapy should be suspected in patients treated with CyX during the previous 6 months before presentation who have bilateral reticular or nodular diffuse opacities on chest x-ray examination or peripheral ground-glass opacities in the upper lung fields on a computed tomographic (CT) scan of the chest. Additionally, a late-onset pneumonitis associated with fibrosis may insidiously develop after months to years of CyX therapy, even with relatively low doses. These late conditions are minimally responsive to corticosteroids, are irreversible, and usually result in terminal respiratory failure or lung transplantation.17
Gonadal Toxicity and Teratogenicity
In addition to minimizing exposure, proposed approaches to fertility preservation include the following: (1) preservation of oocytes, embryos, or ovarian tissue, and (2) the use of depot gonadotropin releasing hormone analogs (GnRH-a) to suppress the metabolism of the ovaries during cytotoxic therapy with CyX. The authors of this text and others have reported favorable results of open trials of depot GnRH-a administration for ovarian protection during monthly IVC therapy. In the authors’ study,18 premature ovarian failure occurred in only 1 of 20 (5%) patients treated with depot GnRH-a, versus 6 of 20 (30%) controls matched for age (mean = 23 years) and cumulative CyX dose (mean = 12 g) (P = <0.05, McNemar test). A metaanalysis has suggested that depot GnRH-a for ovarian protection in women receiving CyX is both safe and effective, and randomized trials are ongoing.19
CyX and its metabolites cross the placenta and appear in breast milk. CyX is a potent teratogen that can cause severe birth defects after administration of as little as 200 mg during early pregnancy. Reported abnormalities included absence of the thumbs, absence of the great toes or all toes, palatal abnormalities, and a single coronary artery.20 Because fertility is preserved in most patients with lupus, highly effective contraceptive techniques, such as intrauterine devices (IUDs), oral contraceptives, or injected progestins in appropriately selected patients, should be strongly considered. The use of CyX in life-threatening lupus during late pregnancy is controversial but may be appropriate in special circumstances because fetal loss is extremely likely when severe maternal flares are uncontrolled. Major CyX-induced toxicities are believed to occur during the first half of pregnancy.
Clinical Trials Administering Cyclophosphamide for Lupus Nephritis
In this discussion, trials of monthly bolus CyX are emphasized, but many modified regimens have been proposed, such as weekly, biweekly, or a once-every-3-weeks bolus CyX given intravenously, and boluses of CyX given orally. These regimens have been reported to be safe and effective in small series. Results of controlled trials are summarized in eTables 50-1 and 50-2. eTable 50-3 summarizes the results of the controlled trials of sequential therapy using CyX for induction of remission.
eTABLE 50-1 Controlled Trials of Cyclophosphamide and/or Azathioprine in the Treatment of Lupus Nephritis
| STUDY | PATIENTS (n) | RESULTS |
|---|---|---|
| Fries and associates, 197323 | 14 | P; then CyX alone |
| Garancis and Piering, 1973134 | 22 | P plus CyX; then P and AZA |
| Donadio and associates 1972, 197460,61 | 26 | More recurrences with P; P versus P plus CyX results in survival with patients on dialysis |
| Ginzler and associates, 197664 | 14 | P plus AZA versus P plus CyX |
| Balow and associates, 198421 | 111 | P plus IVC; then P and AZA and CyX; then P and AZA; then P alone |
| Boumpas and associates, 199222 | 65 | IVC for 30 months; then IVCX for 6 months; then MP alone |
| Sesso and associates, 1994135 | 29 | IVC or MP (both were unsuccessful) |
| Gourley and associates, 1996136 | 80 | IVC; then MP; trend for IVC plus MP and then IVC |
AZA, Azathioprine; CyX, cyclophosphamide; IVC, intravenous cyclophosphamide; MP, bolus methylprednisolone; P, prednisone.
eTABLE 50-2 Controlled Trials Including Bolus Methylprednisolone, Cyclosporin, or Intravenous Immunoglobulin in Systemic Lupus Erythematosus
| AUTHOR | THERAPEUTIC ARMS | RESULTS |
|---|---|---|
| Boumpas and associates, 199222 | Long-term IVC; then short-term bolus MP < either IVC | |
| Sesso and associates, 1994135 | Equivalent outcome; 38% renal failure in 15 months | |
| Gourley and associates, 1996136 | IVC; then bolus MP Trend for IVC and bolus MP; then IVC | |
| Fu and associates, 199880 | Similar renal outcome; 38% renal failure in 15 months | |
| Boletis and associates, 1999137 | Equivalent short-term results |
CS, Cyclosporine; IVC, intravenous cyclophosphamide; IVIG, intravenous immunoglobulin; MP, methylprednisolone; P, prednisone; POC, oral cyclophosphamide.
eTABLE 50-3 Controlled Trials Using Sequential Therapy with Azathioprine or Mycophenolate Mofetil After Induction with Cyclophosphamide in the Treatment of Lupus Nephritis
| Study | Patients (n) | Results |
|---|---|---|
| Chan and colleagues, 200510 | 42 | MMF; then AZA and CyX |
| Houssiau and colleagues, 200235 | 90 | Low-dose IVC and AZA, versus high-dose IVC and AZA |
| Contreras and colleagues, 200426 | 59 | MMF and AZA; less toxic than quarterly IVC and MP |
| Yee and colleagues, 200411 | 32 | IVC plus bolus MP, then followed by AZA; less toxic than POC and bolus MP, then followed by AZA |
AZA, Azathioprine; CyX, cyclophosphamide; IVC, intravenous cyclophosphamide; MP, methylprednisolone; POC, oral cyclophosphamide.
In a seminal 20-year clinical trial at the National Institutes of Health (NIH), patients with proliferative nephritis received either prednisone alone or prednisone plus one of the following: AZA (2 mg/kg/day), AZA (1 mg/kg/day) plus CyX (1 mg/kg/day), CyX (2 mg/kg/day), or bolus IVC for approximately 2 to 4 years.21 Several key findings were revealed: (a) Differences in progression to renal failure were not apparent until more than 5 years had elapsed. After 10 years, however, significant differences in renal survival became apparent, favoring any regimen that included CyX over the administration of prednisone alone. (b) Patients treated with either prednisone alone or with oral CyX had higher death rates than those groups given IVC or AZA plus CyX, which was likely because of the toxicity of daily CyX and the ineffectiveness of prednisone. (c) Oral AZA (1 mg/kg/day) plus CyX (1 mg/kg/day) was equivalent to IVC in terms of preventing renal failure or survival. (d) In serial biopsies, progression of chronic change initially occurred in all patients. Patients who were treated with immunosuppressive agents appeared to stabilize after an initial period of scarring; patients who were treated with prednisone had progressive scarring.
Intravenous Bolus Cyclophosphamide for the Treatment of Lupus Nephritis
Monthly bolus IVC was first described as a treatment for lupus in the 1980s6 and for many years was the standard of care for treating severe lupus. Extensive studies have highlighted the issues discussed in the following text.
Relationship of Efficacy and Toxicity to the Effective Dose of Intravenous Cyclophosphamide
In a retrospective study at the NIH,3 62 patients with proliferative LN treated with CyX were genotyped for common variant alleles of the P-450 enzyme. Homozygosity or heterozygosity for a particular variant allele (CYP2C19*2) predicted not only lower rates of ovarian toxicity, but also a worse clinical response, including an increased risk of end-stage renal disease and the doubling of serum creatinine, suggesting that efficacy and toxicity were both related to the effective dose given. This study provides the best evidence that higher effective doses of monthly IVC increase both therapeutic response and toxicity.
Advantage of Maintenance Immunosuppression Therapy after Intravenous Cyclophosphamide Induction Therapy
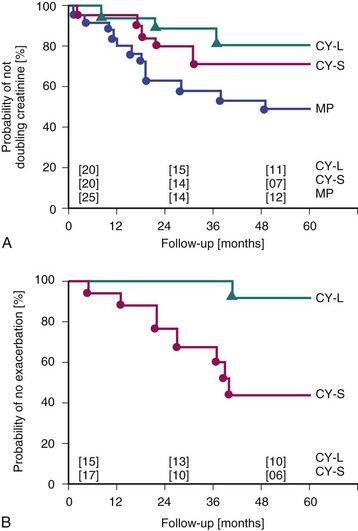
Boumpas and associates22 confirmed early observations that patients treated with monthly IVC for only 6 months had a high rate of subsequent flares. Seven monthly pulses of IVC, followed by an every-3-month IVC maintenance regimen for 2 years, resulted in significantly fewer flares and fewer doublings of serum creatinine, compared with seven monthly pulses of CyX without maintenance pulses (Figure 50-1). The longer IVC regimens, which became generally accepted as the standard of care for LN, resulted in more toxicity, particularly ovarian failure. It has since been suggested that patients who achieve a complete remission after 6 months may have a lower risk of flare. Nonetheless, prolonged immunosuppression (currently using sequential therapy if IVC is initially used) remains appropriate for the majority of patients.
Concomitant Daily Corticosteroids
All published IVC trials have administered daily oral corticosteroids during induction usually beginning with prednisone (1 mg/kg/day) or equivalent . Administering CyX without corticosteroids for LN (e.g., because of a patient’s refusal to take prednisone) is not evidence based and, in the opinion of the authors of this text, unnecessarily exposes patients to a toxic drug using an unproven regimen. One small trial, by Fries and others,23 addressed this issue in 1973 and compared oral CyX alone with prednisone alone for a mean of 9 weeks in 14 patients with lupus and 10 with nephritis. CyX without prednisone failed to control either minor or major manifestations, despite the development of leukopenia and significant additional toxicity. Patients who were changed to prednisone from CyX fared better. These results suggest that CyX and prednisone may act synergistically, and CyX without prednisone may be less effective.
Combining Bolus Methylprednisolone with Intravenous Cyclophosphamide
The initial treatment of LN with bolus corticosteroids (e.g., methylprednisolone [MP] [1000 mg/day] for 1 to 3 days) is widely used (including by the authors of this text), especially in patients with severe disease such as crescentic nephritis or acute renal failure. Serial administration of both agents in combination is supported by an NIH study24 that compared monthly bolus IVC, monthly bolus MP, and the combination of monthly IVC plus bolus MP for LN. During follow-up for a median of 11 years, an intention-to-treat survival analysis revealed the likelihood of treatment failure to be significantly lower in the groups who received CyX (P = 0.04) and combination therapy (P = 0.002) than in the group who received MP alone. Furthermore, the proportion of patients who had doubling of serum creatinine levels was significantly lower in the combination group than in the CyX group. No additional adverse events occurred in the group treated with the combination therapy versus CyX alone, except that patients who received MP pulses had more osteonecrosis.
Racial Differences in Response to Intravenous Cyclophosphamide
Several studies have suggested poorer responses to IVC in African-American versus Caucasian patients. For example, Dooley and associates25 described poorer renal survival in African Americans during the initial period of monthly IVC administration, with several patients rapidly progressing to renal failure, and further disparity appearing during long-term follow-up studies with renal survival after 5 years at 94.5% for Caucasians and 57% for African Americans. These observations are further supported by the results of trials of induction with MMF versus IVC described in the text that follows.
Sequential Therapy for Lupus Nephritis
In a 2004 study by Contreras and colleagues,26 59 patients with LN and the World Health Organization (WHO) class III, IV, or Vb nephritis were treated with monthly IVC for seven doses. Patients were then randomized to maintenance dosing for 1 to 3 years after the initial therapy consisting of quarterly IVC, MMF, or AZA. During maintenance therapy, four deaths occurred in the CyX group and one death was reported in the MMF group; chronic renal failure occurred in three patients in the CyX group, one patient in the MMF group, and one patient in the AZA group. The 72-month, event-free survival, which is defined as no death or progression to hemodialysis, was higher in groups treated with MMF (P = <0.05) and AZA (P = <0.01) versus CyX. The relapse-free survival was also higher in the MMF versus the CyX groups (P = <0.02) (Figure 50-2).
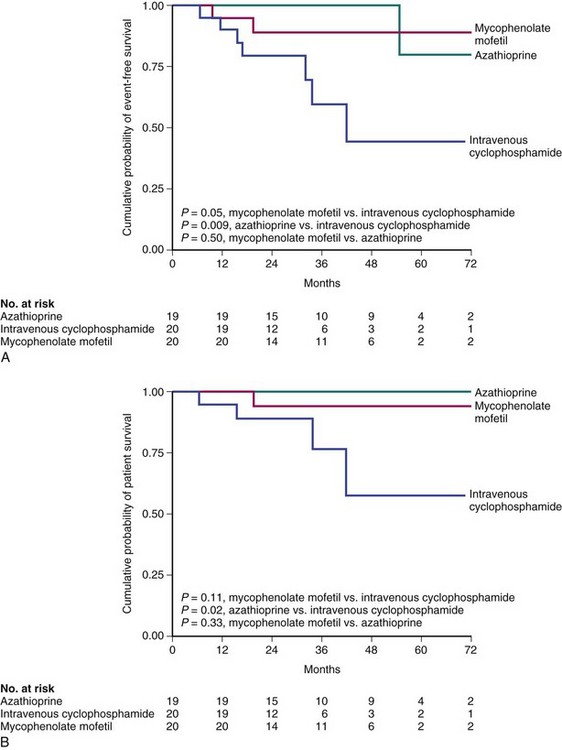
FIGURE 50-2 Mycophenolate mofetil for the treatment of lupus nephritis.
(From Contreras G, Pardo V, Leclercq B, et al: Sequential therapies for proliferative lupus nephritis. N Engl J Med 350:971–980, 2004.)
More recently, patients participating in the Aspreva Lupus Management Study (ALMS) were entered into a maintenance phase study that compared maintenance treatment with either MMF or AZA after patients had completed a randomized trial of induction therapy with IVC versus MMF for LN (see the description provided later in this chapter). MMF was superior to IVC in maintaining remissions (Figure 50-3). The data are consistent with the differences observed in the Contreras trial, which showed a statistically significant advantage of MMF but not AZA over IVC administered every 3 months for maintenance. Interestingly, patients who received MMF for maintenance tended to fare better if they had been randomized to receive IVC rather than MMF as induction therapy in the preceding part of the trial.27
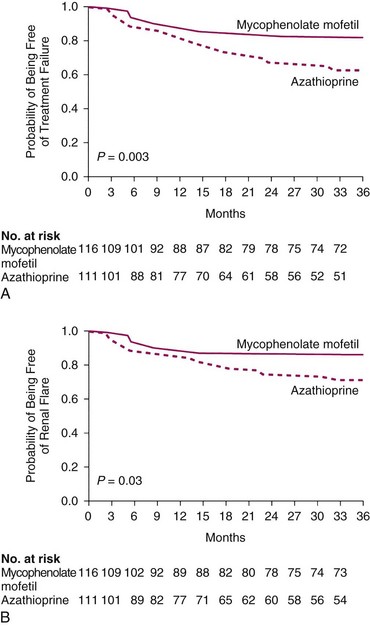
FIGURE 50-3 Kaplan-Meier curves for time-to-treatment failure and time-to-renal flare.
(From Wofsy D, Appel GB, Dooley MA, et al: Aspreva Lupus Management Study maintenance results. Lupus 19:S27, 2010.)
The intention-to-treat population was made up of 227 patients, of whom 116 were given MMF and 111 were given AZA. Figure 50-3 shows the time to treatment failure (see part A) and the time to renal flare from reference (see part B).27
Houssiau and associates,28 on the other hand, did not identify a difference between MMF and AZA as maintenance therapy for LN. Several additional trials have administered daily oral CyX, followed by AZA. For example, Chan and others29 studied 42 patients with somewhat active diffuse proliferative glomerulonephritis who were randomized to daily CyX for 6 months, followed by AZA versus high-dose MMF for 12 months and then by low-dose MMF for 6 months. At long-term follow-up in the MMF group, 81% experienced a complete remission and 14% experienced a partial remission. In the group randomized to CyX and AZA, 76% experienced a complete remission and 14% experienced a partial remission. Oral CyX appeared to be more toxic than MMF.
As previously noted, the EURO-Lupus study28 compared high-dose versus low-dose CyX in patients with lupus and proliferative nephritis; they were then switched to maintenance therapy with AZA.
Another group that used sequential therapy, the European League Against Rheumatism (EULAR), conducted a randomized controlled trial of pulse CyX and MP versus continuous CyX and prednisolone, followed by AZA and prednisolone in LN11; this study suggested that no significant differences were observed between these two regimens. However, enrollment was difficult, and only 32 patients were treated. The authors encountered cytopenias in the group who received oral CyX (2 mg/kg) and concluded, “…the initial dose of 2 mg/kg oral CyX was felt by the investigators to be too toxic to persist with. The intermittent intravenous pulse regimen appears to be better tolerated than oral continuous treatment, with less severe adverse effects.”11
Induction Therapy: Comparisons of Intravenous Cyclophosphamide with Other Agents
Intravenous Cyclophosphamide versus Mycophenolate Mofetil
Chan and colleagues29 compared induction with MMF (2 g/day) with long-term MMF maintenance versus daily oral CyX for 6 months, followed by AZA. Patients were from China and had overall moderately active disease. In the MMF group, 81% experienced a complete remission, and 14% experienced partial remission. In the group randomized to CyX followed by AZA, 76% experienced a complete remission and 14% experienced a partial remission. At long-term follow-up, comparable preservation of renal function and reduction of proteinuria were observed.
Ginzler and associates,30 in a trial that included a high proportion of African-American patients, compared MMF (3 g/day) versus monthly IVC. The higher MMF dose was chosen because of a concern that MMF at 2 g/day was less effective for African Americans than for Caucasians in allogeneic renal transplantation. Patients were required to have creatinine clearances greater than 30 mL/min and serum creatinine levels less than 3.0 mg/dL; overall, they had moderate to very active disease. Patients who did not respond to one regimen were allowed to cross over to the other. At 6 months, the primary endpoint, complete remission, was achieved at a higher rate with MMF than with IVC; however, after 6 months the mean serum creatinine levels and urinary protein excretion were identical when all patients in both groups were considered. A trend revealed that African-American patients responded better to MMF.
Recently, the ALMS trial31 randomized 370 patients with LN to IVC versus MMF. In contrast to the previously mentioned Ginzler trial,30 the overall outcomes in terms of both achievement of remission and serious adverse events and mortality were no different in the two groups. However, as detailed in the following text, a significantly higher response rate to MMF than to was achieved in patients of African-American and mestizo descent and individuals of Hispanic origin.32
The concern that MMF might not be as effective as IVC in preventing the progression of irreversible renal injury in serial biopsies is addressed in two trials. Ong33 compared renal biopsies before and after 6 months of MMF (2 g/day) versus IVC and found comparable reduction of NIH activity scores with somewhat greater increases in chronicity scores in the IVC group than in the MMF group.
Hu34 compared serial renal biopsies in 25 patients treated with MMF versus IVC and showed comparable reductions in activity indices and slight increases in chronicity indices in both groups.
The possibility that brief administration of CyX might be effective in inducing remission was suggested by the Euro-Lupus Nephritis Trial.35 In this study, 90 patients with proliferative glomerulonephritis were randomized to either high-dose CyX (six monthly and two quarterly pulses, increased according to their WBC nadir) or low-dose CyX (six doses of 500 mg CyX every 2 weeks). Maintenance therapy was with AZA. Renal remission was achieved in 71% of patients receiving low-dose CyX versus 54% of patients who were given high-dose CyX; renal flares were observed in 27% of patients in the low-dose group and 29% of those in the high-dose group.
Pulse Cyclophosphamide versus Intravenous Immunoglobulin
Boletis and others36 compared IVC with 10 immunoglobulin infusions and found equivalent results over an 18-month period. Proteinuria actually increased slightly in the IVC group.
Oral Cyclophosphamide
Daily Oral CyX for Induction
As previously noted, Chan and colleagues37 randomized 42 patients to either daily CyX for 6 months, followed by AZA for 6 months, or high-dose MMF for 6 months, followed by low-dose MMF. Complete remissions occurred in 81% of those in the MMF group versus 76% of those in the oral CyX group, suggesting that daily CyX is effective for remission induction although it unfortunately results in three times higher cumulative CyX exposure than IVC exposure for a comparable period. In a second study, long-term outcomes in a cohort of patients with lupus and diffuse proliferative nephritis were studied; this cohort received sequential therapy with oral CyX and prednisolone for induction, followed by AZA for maintenance therapy. Of the 66 patients included in the study, 82.4% achieved complete remission, of whom 39.1% experienced relapse during the follow-up period of 91.7 months, ±36.7 months. No end-stage renal failure or death occurred among the patients, although three patients (4.4%) had doubling of baseline creatinine.
Intravenous Cyclophosphamide in Nonrenal Lupus
Analysis of the recent ALMS trial suggested that nonrenal disease manifestations, in general, responded well to either IVC or MMF and that no significant differences were reported in the responses to one or the other.38
Neuropsychiatric Lupus
Active, steroid-refractory cerebral lupus that is adjudged to be secondary to immunologically mediated injury has responded well to IVC with or without bolus MP in most cases. Anticoagulation has been simultaneously used when distinguishing thrombotic from inflammatory disease has been impossible or to rule out the possibility that vascular inflammation is contributing to the development of thrombosis. Neither the presence of antiphospholipid antibodies nor the involvement of one or more large vessels rules out the use of immunosuppression as opposed to (or in addition to) anticoagulation. Boumpas and associates39 treated nine patients with monthly doses of IVC, three of whom had transverse myelitis and five of whom had focal neurologic findings, seizures, or both. The duration of symptoms ranged from 3 to 45 days. All nine patients had findings suggesting an inflammatory process, including anti-DNA antibodies, and five had cerebrospinal fluid pleocytosis. Five of these patients concomitantly had antiphospholipid antibodies. All patients recovered either partially or completely. These observations suggest that in selected patients who have antiphospholipid antibodies that may not be the major cause of their events, IVC administration is associated with clinical improvement.
Other series of IVC in NP-SLE report favorable results. Neuwelt and others40 retrospectively reviewed 31 patients with NP-SLE who were treated with IVC and in whom a variety of prior therapies had failed, including corticosteroids, warfarin, chlorambucil, and AZA. Indications included organic brain syndrome in 55% of patients, strokes in 35%, peripheral mononeuropathies in 32%, seizures in 29%, and transverse myelitis in 16%. Patients with anticardiolipin antibodies were treated with warfarin. Treatment regimens varied from low-to-high doses of IVC, and plasmapheresis was added in some patients when they appeared not to improve after IVC. Overall, 61% of patients were reported to improve, of whom 26% were not initially improved after 9 months of therapy and appeared to respond to the addition of plasmapheresis. The failure rate for patients with organic brain syndrome was 83%, compared with 37% for other indications. Malaviya and associates41 treated 14 patients with a variety of focal and diffuse neurologic deficits. All patients except the two with seizures stabilized or improved.
Ten patients with bilateral corticosteroid-refractory optic neuritis and severe visual compromise were treated with bolus IVC for 6 months.133 Of the 20 patients, the eyes recovered completely in 10 patients and partially in 6, but the eyes of 4 patients did not recover.
Baca42 treated seven children with NP-SLE (including seizures, focal neurologic deficits, transverse myelitis, and organic brain syndromes) with monthly bolus CyX combined with three initial boluses of MP (30 mg/kg). Three patients had anticardiolipin antibodies but did not undergo anticoagulation. Six patients recovered completely, and one had a minor residual deficit.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree