Evaluation of the Failed Total Hip Arthroplasty
Randy Rizek, Rajiv Gandhi, Khalid Syed and Nizar Mahomed
Key Points
Introduction
Total hip arthroplasty (THA) continues to be one of the most successful orthopedic procedures, improving function and providing significant pain relief among the majority of patients. However, complications do arise postoperatively that can threaten the longevity of the prosthesis. The most common modes of failure following THA include aseptic loosening, infection, dislocation, and periprosthetic fracture. A systematic approach beginning with a thorough history and physical examination aids in determining an accurate diagnosis and the need for a revision procedure.
Clinical Evaluation
Pain is the most common presentation of a patient with a failed total hip arthroplasty (THA). Characterizing the temporal onset, duration, severity, location, and quality of the pain is essential in determining whether the symptoms are due to intrinsic hip pathology or extrinsic causes (Table 85-1). If the patient’s preoperative pain remains unresolved postoperatively in the absence of a pain-free interval, the original diagnosis prior to surgery should be questioned. If the pain differs and is worse postoperatively, the underlying cause is most likely the surgery itself, as in cases of infection, hematoma, component instability, loosening, impingement, or fracture. A delay in the onset of pain postoperatively suggests that the inciting problem may be the implant itself, as can be seen in loosening, chronic infection, or osteolysis.
Table 85-1
Differential Diagnosis of Pain Following Hip Replacement
| Intrinsic Causes | Extrinsic Causes |
| Infection: acute, delayed, late, hematogenous | Lumbar spine disease: stenosis, disk herniation, spondylolysis/spondylolisthesis |
| Aseptic loosening | Malignant tumor: primary, secondary |
| Pain at stem tip (modulus mismatch) | Peripheral vascular disease |
| Greater trochanter nonunion | Metabolic disease |
| Wear debris synovitis | Stress and insufficiency fracture |
| Periprosthetic fracture | Nerve injury: sciatic, femoral, lateral cutaneous |
| Osteolysis | Iliopsoas tendinitis |
| Occult instability | Hernia: femoral, inguinal, obturator |
| Complex regional pain syndrome | |
| Other gastrointestinal, genitourinary, or gynecologic disease |
From Duffy P, Masri BA, Garbuz D, Duncan CP: Evaluation of patients with pain following total hip replacement. Instr Course Lect 55:223–232, 2006.
Determining the location of painful symptoms aids in narrowing the differential diagnosis. Pain localized over the lateral aspect of the hip/thigh may result from trochanteric bursitis, suture/wire irritation, osteolysis, muscle strain, or a fracture. Groin or buttocks pain is typical of vascular or neurogenic claudication, acetabular loosening, or osteolysis. Other causes include iliopsoas impingement and tendinitis due to acetabular retroversion or a prominent acetabular implant.1 Hematoma, various types of hernias, and gynecologic/genitourinary problems are less frequent. Buttocks pain that is associated with radiation distal to the knee is suggestive of spine pathology such as degenerative disk disease or spinal stenosis. Patients with iliopsoas tendinitis describe pain with movements that involve active hip flexion and rotation, such as getting up from a seated position. Thigh pain can be attributable to a loose femoral component, whereby micromotion occurs between the bone and the prosthesis owing to a mismatch in their modulus of elasticity.2,3 However, patients could still have pain despite the presence of a well-fixed cemented or cementless femoral component.
It is important to note circumstances that aggravate and alleviate symptoms. Night pain or pain at rest is suggestive of an underlying infection or malignancy. Painful symptoms associated with activity that are relieved with rest could be due to a loose component, a subtle fracture, or vascular or neurogenic claudication. Pain that is severe with initiating movements, such as getting out of a chair, but resolves once active is associated with early component loosening, spondyloarthrosis, or iliopsoas tendinitis.4 Any history of a traumatic event, such as a sudden slip or fall, preceding the onset of painful symptoms raises the suspicion of traumatic loosening or fracture. Patients with a history of hip and spine pathology may experience increasing pain postoperatively as a result of an increase in their activity levels.
One of the main goals of obtaining a history from a patient with a potentially failed THA is to rule out infection. An infected THA is classified into one of three categories based on onset of symptoms and the underlying cause of the infection.5 Stage I infections occur in the immediate postoperative period, and patients can present with systemic signs of infection such as fever, chills, sweating, and constant pain, along with a red, swollen wound that is draining purulent material. However, this classic presentation of a septic THA is rare.5 Most patients with acute infection of a THA present within the first 12 weeks of surgery with a history of pain and serous wound drainage. The challenge is differentiating whether the infection is superficial or has penetrated the deep fascia to the periprosthetic space.
Stage II infections originate from the index procedure but may involve an acute or delayed low-grade, indolent process. They occur between 6 and 24 months after surgery with increasing pain and a gradually declining level of activity. Pain usually occurs at rest and during weight bearing but is not as severe as with an acutely infected THA. There may be a history of previous infection from another source that preceded the hip symptoms. The patient must be questioned regarding whether there was a delay in the discharge, prolonged use of antibiotics, night pain, or a history of persistent wound drainage.
Stage III infections are the least common and the easiest to diagnose. The clinical presentation consists of sudden onset of hip pain without a history of perioperative sepsis. Pain on weight bearing or any movement of the hip along with rest pain is characteristic of a late deep infection after the patient has been asymptomatic for 2 years or longer after surgery. It occurs as the result of hematogenous spread from another remote source. Patients usually can recall a previous dental procedure, respiratory infection, genitourinary procedure or infection, or open skin lesion before the onset of symptoms. However, medications such as antibiotics or steroids can mask these symptoms. Immunocompromised patients, intravenous (IV) drug users, and patients who require frequent urinary catheterizations are at high risk for stage III infection.
Clinical assessment of a patient with a problem following THA includes determining the presence of subluxation or dislocation. Patients will describe a sensation of popping or clicking, suggesting that the hip is coming into and out of the joint. They can also present with an acute dislocation precipitated by a traumatic event. Most dislocations occur within the first year of surgery.6 Patient factors such as gender, body habitus, a history of neuromuscular or cognitive disorders, and alcohol use are shown to increase the rate of dislocation.7 Any previous history of hip surgery and the surgical approach utilized should be determined. In the setting of acute dislocation, information surrounding the episode, including any history of previous dislocations, is crucial in elucidating the cause of instability.
Identifying the patient’s functional status requires a thorough evaluation to assess the impact that the problematic THA is having on daily activities. Several standardized scales are available to assist in quantifying a patient’s level of disability and pain; these include the Western Ontario and McMaster University (WOMAC) Osteoarthritis Index and Short Form (SF)-36.8 These scales can also be useful in assessing postoperative improvement.
The patient’s medical history and a review of systems should be obtained. Most often, THA is performed on members of the elderly population, who are likely to have comorbidities that predispose them to postoperative complications. Risk factors for infection as discussed previously should be ruled out. Any history of venous ulcers or vascular insufficiency may necessitate a vascular surgery consultation. If the patient has been immobilized postoperatively, the risk of developing a pulmonary embolism and deep vein thrombosis is high, requiring appropriate workup. A positive cardiac history will require preoperative assessment to ensure medical optimization and to determine whether surgical intervention is a viable treatment option. Patients who have received previous pelvis irradiation treatment with radiation osteonecrosis are not suitable for porous-coated implants if revision hip surgery is required.9 Previous hip operations should be documented. The presence of constitutional symptoms should not be overlooked, thereby avoiding the danger of missing an underlying infection or malignant process.
Finally, an attempt should be made to obtain all operative records from the primary surgery along with the implant labels. These provide insight into any technical challenges that may have arisen. They also describe the type and size of implant that was used, providing information that is invaluable in preoperative planning and assessment of compatibility if revision surgery is indicated.
Physical Examination
A complete musculoskeletal examination must be performed when a patient presents with a problem following THA. The contralateral hip, both knees, and the lumbar spine are incorporated into the routine examination to rule out pathology from referred sources. The examination should begin with an assessment of the patient’s gait, which can help identify a leg length discrepancy, antalgia, or abductor weakness. A leg length discrepancy (LLD) of 2.5 cm or greater can cause a limp with a vaulting-type gait pattern, but its effect on mechanical failure or correlation with the onset of low back pain is inconclusive.10 Although its functional implications are not well defined, a substantial LLD is dissatisfying to the patient and is a common reason for litigation. It must be determined whether it is a true or apparent LLD. True leg length can be measured from the anterior superior iliac spines to the medial malleoli of the ankles. A true bony inequality exists if the two measurements are unequal. The LLD should be compared with measurements taken postoperatively because increasing discrepancy would suggest gradual subsidence of the components.11 An apparent leg length discrepancy is identified by measuring the distance from the umbilicus to the medial malleoli. In the absence of a true LLD, an apparent LLD can occur owing to pelvic obliquity, adduction, or flexure contracture.
A Trendelenburg gait can be observed when the abductor muscles are weak or nonfunctional, whereby the unsupported hip drops during the midstance phase of the gait cycle, and the patient exhibits a characteristic lurch to compensate for the instability. The abductors can be tested against gravity by having the patient abduct his leg while in the lateral decubitus position.
The skin should be carefully examined with particular attention to the condition of the primary incision and its usefulness for revision surgery. Inspection for signs of inflammation, persistent drainage, or healed sinus tracks should be documented. Palpation can aid in localizing the source of symptoms in that tenderness along the scar suggests a possible neuroma. The greater trochanter, femur, and pubic rami should be palpated to rule out a possible trochanteric bursitis, occult fracture, or metastatic deposit. The iliac fossa and inguinal region should be examined for fullness or masses that may suggest the presence of hernias.
The hip and adjacent joints should be taken through full active and passive ranges of motion. Pain at the extremes suggests component loosening, and pain with any form of movement may indicate an infection or an inflammatory process. Impingement or instability will cause pain in a particular position or movement. Pain caused by trochanteric bursitis, gluteal calcific tendinitis, and heterotopic ossification can be exacerbated by resisted abduction. Pain with resisted hip flexion or passive hip extension may suggest iliopsoas tendinitis,1 and pain with passive straight-leg raise is indicative of a lumbar spine radiculopathy.
A detailed neurovascular examination is essential in ruling out neurogenic or vascular causes of symptoms. Patients with a history of spine pathology are at increased risk for nerve palsy.12 Preoperative documentation of any motor or sensory deficits should be obtained. Direct nerve injury can result from surgical trauma, traction, retractors, limb lengthening, positioning, or thermal or pressure injury from cement.10 The peroneal division of the sciatic nerve is the most commonly injured nerve, as is demonstrated by weakness in ankle dorsiflexion and decreased/loss of sensation to the dorsum of the foot.13 This should be carefully assessed in the setting of limb lengthening. Injury to the tibial division is rare but is seen with weakness of the knee flexors and ankle plantarflexors.14
The vascular integrity of the limb should be closely scrutinized. Any sign of vascular compromise or insufficiency should warrant further investigation. A swollen, tender calf should raise suspicion of a deep venous thrombosis, and incisions from a previous vascular bypass surgery may necessitate a vascular consultation.
Further abdominal, pelvic, and rectal examinations may be warranted in an effort to rule out causes of referred pain.
Imaging of the Failed Total Hip Replacement
Evaluation of the failed total hip replacement (THR) requires knowledge of diagnostic imaging options. These imaging tools have evolved in diagnostic accuracy, and the choice of test ordered by the evaluating physician must reflect some understanding of the sensitivity and specificity of these tests. As a reminder for the reader, the sensitivity of a test represents its true positive rate; specificity refers to the true negative rate.
Standard evaluation of the failed THR should include an anteroposterior (AP) view of the pelvis centered on the pubic symphysis, an AP view of the affected hip, and a shoot-through lateral of the affected hip. Radiographs should cover the full extent of the prosthesis, including the entire column of cement if a cemented femoral component exists. In addition to alignment and positioning of the components, areas of osteolysis, femoral bowing, cement mantle fractures, and cortical thinning can be seen. Positioning the limb in 15 to 20 degrees of internal rotation allows for an accurate assessment of the femoral neck shaft angle and offset. Measurement of the femoral canal diameter can be difficult with magnification errors; however, standardization with marker films can be helpful.
Patients with pelvic and acetabular osteolysis or post-traumatic deformities may be assessed with Judet views to evaluate the integrity of the anterior and posterior columns. These radiographs should be reviewed for heterotopic ossification, and those with Brooker grade 3 or 4 disease should be considered for prophylaxis following revision surgery. This prophylaxis may consist of postoperative radiation or oral indomethacin.
Clinical examination and imaging of a painful metal-on-metal bearing hip replacement must consider the possibility of an inflammatory “pseudotumor.” This pseudotumor signifies a high-grade inflammatory reaction in the soft tissues, and although the exact cause is unclear, it is believed to be mediated by a lymphocytic reaction to local cobalt and chromium ions. Patients with these reactions may present with regional hip pain, a palpable soft tissue mass, spontaneous dislocation, or associated nerve palsies.15,16
Ultrasound is likely the best imaging modality of choice if a pseudotumor is first suspected. Magnetic resonance imaging findings can include a soft tissue mass with muscle necrosis and cystic fluid collections. X-ray findings may show an elevated acetabular cup abduction angle because this is believed to be associated with elevated ion levels. The best reported treatments are pseudotumor excision and a change in the bearing surface.15,16
Osteolysis and Wear
Osteolysis is a major reason for failure following THA. It is a biological process caused by particle debris that leads to bone loss around the prosthesis. The cause is multifactorial and is influenced by patient, implant, and surgical factors. The terms osteolysis and aseptic loosening have been used interchangeably in the literature, but they refer to the same biological process occurring at the metal-bone or cement-bone interface. Clinical manifestations of osteolysis can range from slowly progressive radiolucencies around a previously well-fixed component that can result in mechanical loosening to rapidly expansile radiolucent lesions that may or may not result in mechanical loosening. Therefore, it is essential for orthopedic surgeons who perform THA to be aware of the clinical and radiographic patterns of osteolysis.
Causes
The osteolytic process is initiated by particulate debris generated from wear sources, most of which come from the bearing surfaces of the implant. Wear debris can consist of polyethylene, polymethylmethacrylate, metals, or ceramics. The size, shape, and concentration of these particles influence the extent of osteolysis that occurs. Wear has been shown to consist mostly of submicron polyethylene particles generated from a cobalt-chromium-polyethylene articulation.17 These particles are known to illicit an enhanced host immune response, whereby macrophages ingest particle debris, leading to activation of an inflammatory cascade. These inflammatory markers initiate osteoclast activity and inhibit osteoblast activity, which leads to the resorption of bone. Because this process compromises the metal-bone or cement-bone interface, micromotion of the implant occurs; this can lead to the production of additional wear debris and eventual mechanical loosening.
The extent of osteolysis and loosening is influenced by particle access to the prosthesis interface. Particles can travel within the new prosthetic joint and in the potential space around the prosthesis-bone interface, which is referred to as the effective joint space.18 Access to the effective joint space is influenced by implant factors such as shape, size, and extent of porous coating.
Risk Factors
Specific factors that place a patient at increased risk of osteolysis include age, male gender, and high levels of activity. Young patients have been specifically shown to have higher rates of acetabular loosening and osteolysis, but age does not influence the rate of femoral loosening.19 The generation of wear debris is generally higher among more active individuals and is not associated with a patient’s weight.20,21 It is thought that aerobic activity is well tolerated as opposed to high-impact activities such as running or repetitive heavy lifting.20
Implant-related factors are known to effect the generation of wear debris. Polyethylene sterilized by gamma irradiation in a vacuum rather than in air demonstrates increased fatigue strength and wear resistance. Oxidation of polyethylene in an inert atmosphere leads to cross-linking between polymer chains.22 Highly cross-linked polyethylene has been shown to generate lower rates of wear debris.23 Polyethylene thickness less than 6 mm is associated with higher rates of osteolysis.24 Alternate bearing surfaces have been introduced to increase the longevity of THA and have demonstrated improved wear characteristics. Hard surfaces such as ceramic articulating with cross-linked polyethylene or ceramic demonstrate minimal amounts of osteolysis.17,25 Modern metal-on-metal bearings generate a lower volume of wear and smaller particulate debris, but the long-term effects of metallosis remain unknown. Other surgical factors associated with increased wear rate include malalignment of the acetabular component and failure to restore femoral offset.26
Diagnosis
Osteolysis can exist in the absence of clinical symptoms, making the diagnosis challenging. The patient may remain asymptomatic despite evidence of periprosthetic radiolucencies or enlarging focal defects on plain radiographs. It is recommended that serial radiographs be performed during the first 5 to 7 years of follow-up to screen for evidence of ingrowth and the presence of radiolucencies or loosening. Computed tomography (CT) scans should be utilized to screen young, active patients following THA for evidence of osteolysis.
Patients typically begin to present with pain when bone loss leads to implant loosening or a periprosthetic fracture. Pain localized to the thigh is usually associated with femoral loosening, and groin or buttock pain is associated with acetabular loosening. Pain occurs with activity and can be present at night. The presence of a periprosthetic infection should always be considered in cases of failed THA, because the signs and symptoms mimic those of aseptic failure. Other less common causes of femoral osteolysis should also be considered, such as metastatic carcinoma, multiple myeloma, lymphoma, stress shielding, and premature cement mantle fracture.22
Radiology and Classification
Femoral Component
To evaluate for aseptic loosening of cemented femoral components, the categories of definitely loose, probably loose, and possibly loose should be used. Definitely loose is defined as migration of the femoral stem, a new continuous lucent line at the stem-cement interface, a stem fracture, or fracture of the cement mantle.27 Probably loose is defined as a continuous lucent line at the cement-bone junction. Possibly loose is defined by a radiolucent line between 50% and 100% of the cement-bone interface. It should be noted that cement-bone lucencies are commonly seen, and although they may represent aseptic loosening, they also may be due to nonfilling of the canal with cement, remodeling, and neocortex formation. Aseptic loosening demonstrates progressive and localized endosteal scalloping on subsequent films; neocortex formation with remodeling appears as a nonprogressive linear radiolucency. The four modes of failure of cemented femoral stems were described by Gruen as pistoning, medial midstem pivot, calcar pivot, and bending cantilever fatigue.28 Gruen divided the femoral component into seven radiographic zones where radiolucency can develop in cemented or uncemented stems (Fig. 85-1). Progressive radiolucency within these zones is indicative of femoral loosening.
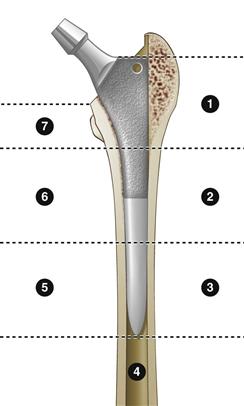
Figure 85-1 Division of the femoral prosthesis into the seven Gruen zones.
Uncemented femoral stems that are well fixed are characterized by the absence of radiolucent lines adjacent to the porous coating and the presence of spot welds. Cortical atrophy and stress shielding may be seen just proximal to these spot welds.3 Any subsidence of an uncemented implant as seen by an increase in vertical distance between the top of the greater trochanter and the shoulder of the prosthesis is generally considered a sign of instability. A bony pedestal is an endosteal condensation of bone that may or may not extend across the intramedullary canal. It is considered evidence of lack of bony ingrowth as the stem attempts to obtain vertical stability. A bony pedestal at the distal tip would likely not occur in a bony ingrown stem because with a stable stem, stress transfer to bone occurs at the level of the proximal porous coating.
Temmerman and associates examined the utility of plain radiography, subtraction arthrography, nuclear arthrography, and bone scintigraphy in diagnosing aseptic loosening of the femoral component. They concluded that both bone scintigraphy and nuclear arthrography made a significant contribution to the diagnosis beyond that made by plain radiography alone.29
Use of magnetic resonance imaging (MRI) in the diagnosis of aseptic loosening of uncemented femoral stems has been evaluated in a few studies. One group found that high signal on T1 and short tau inversion recovery (STIR) MRI was associated with radiographic signs surgical and pathologic findings of loosening.30 MRI with metal artifact reduction techniques has been demonstrated to be a valuable tool in diagnosing abductor tendon avulsion injury following hip arthroplasty.31
Radiographic evaluation of a second-generation metal-on-metal bearing surface total hip replacement warrants special consideration here. Particle-induced osteolysis is considered a major cause of aseptic loosening in cemented and uncemented metal-on-polyethylene total hips; however, this disease may also occur in metal hips. Beaule and colleagues reported on a case of progressive osteolysis at the distal tip of the femoral stem in an uncemented second-generation metal-on-metal total hip that required revision surgery.32 Similarly, other authors reported on 10 hips with evidence of osteolysis around a metal-on-metal bearing total hip replacement.33 These authors suggest that the cause was an immunology response to metal ions in the periprosthetic soft tissue. Specifically, they point to antigen-specific sensitization of T cells as a mediator of this process.33 Most reported cases of osteolysis around these implants occur on the femoral side.
Acetabular Component
The diagnostic evaluation of a loose acetabular component is generally considered more difficult than evaluation of the femoral component.34 Reported imaging techniques include plain radiographs, subtraction arthrography, bone scintigraphy, and CT.
Plain radiographs can be used to assess acetabular cups for aseptic loosening based on the zones defined by DeLee and Charnley.35 Loosening of cemented acetabular components usually begins at the cement-bone interface. Cups with greater than 2 mm lucency in all three zones or in zones 1 and 2 are likely to be loose. Any cups that demonstrate signs of migration are considered definitely loose. Radiolucent lines around cementless acetabular cups are less reliable for loosening than those around cemented acetabular cups. Definite signs of loosening include migration of the cup, screw breakage, fracture of the shell, and shedding of the porous surface.
When an osteolytic defect is evaluated, plain radiographs have the limitation of trying to evaluate a three-dimensional defect with two-dimensional imaging. The AP view of the hip provides good information about the dome and floor of the acetabulum; however, it can be difficult to distinguish between osteolysis of the anterior and posterior columns.36 Judet views may be helpful for better evaluating the acetabular columns. Many studies have shown that plain radiographs alone underestimate the size and number of acetabular defects.37,38 A systematic review and meta-analysis of this topic concluded that the sensitivity and specificity of plain radiographs alone in detecting aseptic loosening of acetabular cups were 70% and 80%, respectively.39 Three commonly used classifications for acetabular bone defects are the American Academy of Orthopedic Surgeons (AAOS) classification, the Paprosky classification, and gross classifications (Boxes 85-1 through 85-3). When these classifications were reviewed with the use of plain radiographs, intraobserver and interobserver reliability was found to be low.40









