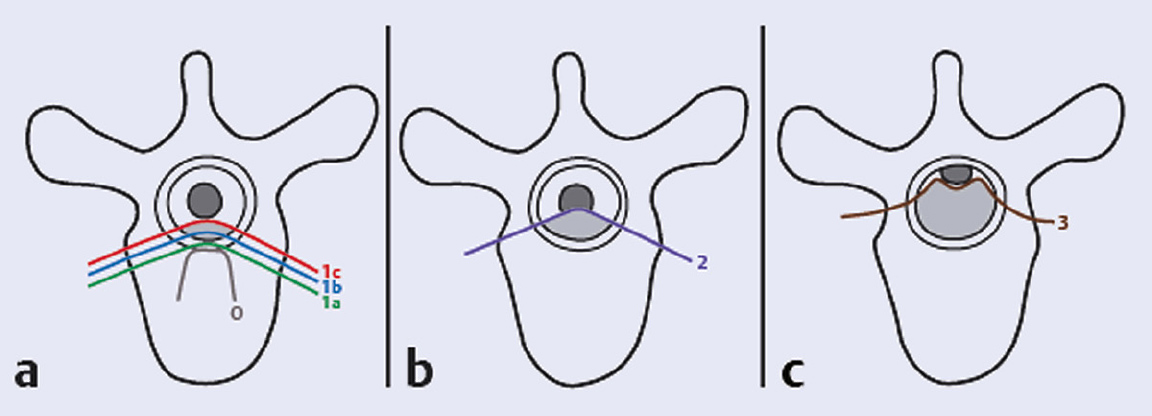
1 Every year, more than 1.6 million new cases of cancer are diagnosed in the United States.1 Roughly half of these patients eventually die from their disease, frequently due to complications from metastasis. The bone is the third most common site of metastases following lung and liver,2 and the spine is the most common site for bone metastasis. As many as 30 to 70% of cancer patients are found to have spinal metastases on autopsy studies.3 Symptomatic secondary metastases are estimated to occur in approximately 10 to 20% of all cancer patients.3,4 The highest incidence of spinal metastases is found in individuals 40 to 65 years of age, corresponding to the period of highest cancer risk; 5 to 10% of patients with cancer develop spinal cord compression.3 Up to 50% of spinal metastases require some form of treatment, and 5 to 10% require surgical management.5 Moreover, as survival rates for many primary cancers continue to improve, it is likely that the prevalence of spinal metastases will increase. The common tumors that spread to the spine in adults are breast, lung, prostate, renal, melanoma, thyroid, and colorectal cancers, as well as hematologic malignancies.6–9 Multiple myeloma has the highest tendency for spinal metastases of all tumors. Spine tumors in children are represented by various forms of neuroblastoma and sarcomas.7 There are several ways in which tumors disseminate to the spine including hematogenous spread, direct extension or invasion, and seeding of the cerebrospinal fluid. The thoracic spine is the most common site of involvement (70%), followed by the lumbar spine (20%), cervical spine, and sacrum. The vertebral body is involved in 80% of cases, with the posterior elements being affected in 20%. Most metastases are osteolytic (95%), with breast and prostate carcinomas accounting for most of the osteoblastic metastases. Occasionally both osteoblastic and osteolytic metastases occur in the same patient. Almost invariably, metastatic tumors do not involve the dura (i.e., they are epidural), but certain sarcomas and recurrent metastatic tumors after radiotherapy can violate the dural barrier. With improvements in chemotherapy and hormonal therapy, and with the advent of novel targeted agents, medical oncologists have an increased number of therapeutic options and survival times have improved over the years. Radiotherapeutic techniques have also evolved. Spinal stereotactic radiosurgery and intensity-modulated radiation therapy (IMRT) techniques are enabling the delivery of high-dose conformal radiation to spinal tumors, erasing some of the distinction between radiosensitive and radioresistant tumor histologies. Lastly, advances in surgical techniques now enable the surgeon to treat spinal metastases more effectively than before. Spine surgery can correct mechanical instability, relieve neurologic compression, and improve pain.9,10 Increasingly, this can be achieved through minimally invasive techniques that entail less morbidity and enable faster recovery.11,12 The increasing number, and complexity of, treatment modalities available for patients with spinal metastases can complicate the decision making, so the evaluation of these complex patients must be multidisciplinary, and the decision to perform surgery must be based on four key aspects of the patient’s status: medical fitness, clinical presentation, oncological status, and feasibility of surgical treatment. This evaluation scheme is detailed in the remainder of this chapter. It is not meant to be an algorithm but rather a consideration of these four key aspects when developing a treatment plan for the patient with spinal metastasis and when determining the role of surgery. The first fundamental consideration in managing patients with metastatic spinal disease is their overall medical condition. Many cancer patients have received prior chemotherapy or radiation, as well as steroids, and they may be malnourished from their treatment or from the disease. This may have an impact on their ability to tolerate surgical intervention. General patient factors such as overall health, nutritional status, and medical comorbidities should all be considered in deciding whether to recommend surgery.10 Patient factors that have been found to be related to poor surgical outcome include advanced age, obesity, malnutrition, diabetes, low bone mineral density, chronic corticosteroid use, and bone marrow suppression.13 Hematologic status, such as leukopenia, thrombocytopenia, or coagulopathy—conditions common among cancer patients receiving chemotherapy or radiation therapy—must also be considered. As a general rule, the more extensive the surgical procedure, the healthier the patient needs to be in order to survive the surgery and enjoy durable benefits. The patient’s medical fitness may be considered not only in deciding whether to recommend surgery or not, but also in the selection of the appropriate surgical procedure and approach. Nonsurgical treatments, such as conventional radiation therapy or radiosurgery, or minimally invasive spinal procedures, such as percutaneous vertebral augmentation, may be appropriate for patients with significant medical risks or limited prognosis. The second key consideration in the management of metastatic spinal disease is the patient’s clinical presentation. Patients with spinal metastases typically present with neurologic symptoms, pain, or signs of mechanical instability. It is important to recognize that the nature of the clinical presentation and the severity of the clinical findings have an impact on the choice of treatment modality. Neurologic dysfunction is a common finding in patients with metastatic disease of the spine. A careful neurologic assessment must look for sensory and motor disturbance, autonomic dysfunction, as well as long tract signs. The main focus of the neurologic assessment is on localizing the potential lesion and determining the clinical extent of the myelopathy or the functional radiculopathy. This clinical information is then combined with the radiological evaluation to assess the degree of epidural spinal cord compression (ESCC) or nerve root compression. Approximately 5 to 10% of patients with metastatic spine tumors develop metastatic epidural spinal cord compression (MESCC). Historically, treatment of MESCC by decompressive laminectomy alone did not provide substantial clinical benefit beyond that of conventional radiation, and it frequently further compromised spinal stability.14,15 The development of improved surgical approaches and spinal instrumentation to treat instability has resulted in better surgical techniques for spinal decompression and stabilization.4 Using techniques of circumferential decompression and stabilization, Patchell et al10 conducted a randomized prospective trial of 101 patients with MESCC. They conclusively showed that early surgical decompression and stabilization followed by postoperative radiotherapy is superior to treatment with radiotherapy alone for patients with spinal cord compression caused by metastatic cancer. It should be noted that this study did not include patients with highly radiosensitive/chemosensitive tumors such as myeloma, lymphoma, and small cell lung cancer. Significantly more patients in the surgery group (84%) than in the radiotherapy group (57%) could ambulate after treatment (odds ratio [OR], 6.2; 95% confidence interval [CI], 2.0–19.8; p = 0.001). These patients were able to maintain their ambulation for a greater duration—a median of 122 days in the surgical group compared with 13 days in the radiotherapy group (p = 0.003). The need for corticosteroids and opioid analgesics was significantly reduced in the surgical group. The degree of MESCC at the time of clinical presentation is highly variable amongst patients. The selection criteria in the Patchell study included only patients with true deformation of the spinal cord. The Spine Oncology Study Group (SOSG) has developed and validated a six-point grading system to describe the degree of ESCC based on axial T2-weighted magnetic resonance imaging (MRI) at the site of most severe compression (Fig. 1.1).16 This radiological assessment can be used in combination with the neurologic examination and tumor histology to help guide treatment. For patients with highly radiosensitive or chemosensitive tumors, nonsurgical treatment may be adequate even in cases of high-grade spinal cord compression due to the rapid response of these tumors to radiation or chemotherapy. For other solid metastatic tumors, the Patchell study suggests that high-grade (grade 2 or 3) MESCC is best treated with surgical decompression and stabilization followed by radiation therapy. For patients who do not have significant myelopathy or functional radiculopathy with low-grade MESCC (grade 1c or less), surgery may not be necessary (unless there is significant spinal instability; see below). In this case chemotherapeutic or radiotherapeutic options (including spinal radiosurgery for radioresistant histologies) can be utilized. The nature and severity of the neurologic and radiological findings clearly influence the choice of treatment.17 Metastatic spine tumors most commonly come to attention with the development of pain. This occurs in 83 to 95% of patients and typically precedes the development of other neurologic symptoms.18 It is important to recognize that there are different types of pain caused by metastatic spine tumors, and the nature of the pain may impact decision making. There are three types of pain that affect patients with symptomatic spinal metastases: local or biological, radicular, and mechanical. Fig. 1.1a–c Schematic representation of the 6-point epidural spinal cord compression (ESCC) grading scale16. Grade 0, tumor is confined to bone only. (a) Grade 1, tumor extension into the epidural space without deformation of the spinal cord. This is further divided into 1a, epidural impingement but no deformation of the thecal sac; 1b, deformation of the thecal sac without spinal cord abutment; and 1c, deformation of the thecal sac with spinal cord abutment but no compression. (b) Grade 2, spinal cord compression but cerebrospinal fluid (CSF) is visible. (c) Grade 3, spinal cord compression without visible CSF. Grades 0, 1a, and 1b are considered for radiation as first treatment in the lack of mechanical instability. Grades 2 and 3 define high-grade ESCC. Note: Used with permission from Bilsky MH, Laufer I, Fourney DR, et al. Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine 2010;13(3):324–328. Local pain is thought to result from periosteal stretching, elevation of endosteal pressure, or inflammation caused by tumor growth.19 The pain can be localized, is often constant, and presents in the evenings and mornings. It is described as a deep “gnawing” or “aching” pain at the site of disease; it does not worsen with movement and may improve with activity. This type of pain is quite responsive to anti-inflammatory or corticosteroid medications, and radiation therapy can relieve it by shrinking the tumor and decreasing the production of inflammatory mediators. Radicular pain is caused by nerve root impingement, which occurs when a spinal metastases compresses the exiting nerve root within the spinal canal, within the neuroforamen, or outside the foramen. The radicular pain follows a dermatomal distribution and is described as sharp, shooting, or stabbing in nature. It is often constant and may or may not be relieved with changing position. As with local pain, radicular pain may respond to therapies that can reduce the effective size of the tumor, including corticosteroids, chemotherapy, and radiation therapy. Mechanical pain is severe and movementrelated. It typically worsens with loading of the spine as a patient moves from lying down to sitting and from sitting to standing. Bending often exacerbates the pain, and it is relieved with recumbency. It is typically associated with vertebral collapse, as the weakened vertebra is no longer able to support the mechanical loads placed on it. It is important when assessing spine tumor patients to evaluate their pain when they are sitting or standing, because mechanical pain might not be noted in patients lying in bed. Mechanical pain must be distinguished from local and radicular pain. It is often refractory to anti-inflammatory medications, chemotherapy, and radiation. Although these modalities may treat the underlying tumor, they are not effective at restoring the mechanical integrity of the spine, and therefore are unlikely to provide durable relief of mechanical pain. Patients with mechanical pain typically require a treatment aimed at strengthening the affected spinal region, such as cement augmentation or spinal stabilization. The final component of the clinical evaluation is to recognize the patient presenting with spinal instability. The SOSG has defined neoplastic spinal instability as the “loss of spinal integrity as a result of a neoplastic process that is associated with movement-related pain, symptomatic or progressive deformity, and/or neural compromise under physiologic loads.”20 Mechanical instability due to spinal metastasis is an indication for surgical stabilization or percutaneous vertebral augmentation, regardless of the ESCC grade or the chemo/radiosensitivity of the tumor. Although effective for local tumor control, chemotherapy and radiation therapy have little or no impact on spinal stability. As a result, patients with gross neoplastic spinal instability generally require a surgical intervention. The assessment of spinal instability is based on a combination of both clinical and radiographic information. The SOSG has proposed a set of criteria, the Spine Instability Neoplastic Score (SINS) (Table 1.1),20 to help clinicians evaluate instability. This grading scheme is based on six parameters: location of the lesion, presence and type of pain, radiographic alignment, nature of the lesion (lytic or blastic), vertebral body collapse, and posterior element involvement. Each parameter receives a numerical score. Metastatic spine lesions with a low SINS (0–6) are generally considered stable and do not require surgical intervention, whereas a high SINS (13–18) suggests instability that is likely to require surgical stabilization. Intermediate SINS (7–12) tumors are considered potentially unstable and represent the middle of the instability spectrum. The SINS does not recommend any specific treatment but is a guide to help both surgeons and nonsurgeons recognize those patients who might be at risk for progressive vertebral collapse and deformity. The SINS demonstrated near perfect interand intraobserver reliability in determining the three clinically relevant categories of stability: stable (SINS 0–6), potentially unstable (SINS 7–12), and unstable (SINS 13–18). The sensitivity and specificity for detecting potentially unstable or unstable lesions were 95.7% and 79.5%.13 Table 1.1 The Spinal Instability Neoplastic Score (SINS)
Evaluation and Decision Making for Metastatic Spinal Tumors
 Introduction
Introduction
 Medical Fitness
Medical Fitness
 Clinical Presentation
Clinical Presentation
Neurologic Function
Pain
Mechanical Instability
SINS Component | Description | Score |
Location | Junctional (occiput-C2, C7-T2, T11-L1, L5-S1) | 3 |
| Mobile spine (C3-C6, L2-L4) | 2 |
| Semi-rigid (T3-T10) | 1 |
| Rigid (S2-S5) | 0 |
Paina | Yes | 3 |
| Occasional pain but not mechanical | 1 |
| Pain-free lesion | 0 |
Bone lesion | Lytic | 2 |
| Mixed (lytic/blastic) | 1 |
| Blastic | 0 |
Radiographic spinal alignment | Subluxation/translation present | 4 |
| De novo deformity (kyphosis/scoliosis) | 2 |
| Normal alignment | 0 |
Vertebral body collapse | > 50% collapse | 3 |
| < 50% collapse | 2 |
| No collapse with > 50% body involved | 1 |
| None of the above | 0 |
Posterolateral involvement of spinal elementsb | Bilateral | 3 |
| Unilateral | 1 |
| None of the above | 0 |
aPain improvement with recumbency and/or pain with movement/loading of the spine.
bFacet, pedicle or costovertebral joint fracture or replacement with tumor.
Source: From Fisher CG, DiPaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine 2010;35:E1221–E1229. Reproduced with permission.
 Oncological Status
Oncological Status
The third key element of the evaluation of the patient with spinal metastatic disease is oncological status. Most importantly this includes recognition of the specific tumor histology. In addition, the extent of metastatic disease (bone, visceral) and the extent and nature of prior treatment also have an impact on the management of the spine metastasis.
Histology
It is critical to identify the tumor histology because it provides important information about the patient’s prognosis. In fact, the histology of the primary tumor is the single strongest predictor of postoperative survival in patients undergoing surgery for spinal metastases.21,22 According to Tomita et al,21 tumor histologies can be stratified into three groups: slow-growing tumors, including breast, prostate, thyroid, and carcinoid tumors; moderately growing tumors, including those arising from the kidney and uterus; and rapidly growing tumors, including tumors of the lung, liver, stomach, esophagus, pancreas, bladder, sarcoma, and tumors of unknown origin. In general, the more aggressive the histology, the worse the prognosis.
Knowledge of the tumor histology also provides critical information regarding the responsiveness of the spinal metastasis to nonsurgical therapies such as chemotherapy and radiation therapy. The impact of chemotherapy and radiotherapy varies considerably with tumor type.23,24 Chemotherapy is generally reserved for asymptomatic or minimally symptomatic lesions because its effects typically take time to manifest and this may be problematic for symptomatic patients. The obvious exceptions to this are the hematologic malignancies or Ewing’s sarcoma, which may respond rapidly to chemotherapy. In patients without neurologic deficit or spinal instability, it may be perfectly reasonable to utilize systemic therapy for sensitive histologies. Breast and prostate cancers, for example, can be quite sensitive to hormonal therapies.25,26
With regard to radiation, tumors have traditionally been considered radiosensitive or radioresistant depending on their response to conventional radiation therapy (CRT).23,24 With-out precise conformal technique, the dosing of CRT is limited by spinal cord tolerance, as the cord is within the radiation field that is used to treat the tumor. Hence, a tumor that responds favorably to doses limited by cord tolerance is considered radiosensitive, whereas those that do not respond favorably to these doses are considered radioresistant. In cases where the tumor is in close proximity to the spinal cord, it may be impossible to radiate the tumor without radiating the cord, and the radiosensitivity of the tumor, therefore, will determine whether this modality can be used effectively. Most authors consider lymphoma, myeloma, and seminoma to be highly radiosensitive and treatable with CRT even in cases of spinal cord compression. Of the solid tumors, breast, prostate, ovarian, and neuroendocrine carcinomas are considered to be radiosensitive, whereas renal, thyroid, hepatocellular, non–small-cell lung, colon, melanoma, and sarcoma are considered radioresistant.17 Spinal stereotactic radiosurgery (SRS), which can be used to deliver highly conformal doses of radiation to spinal tumors while avoiding the spinal cord, has been shown to provide excellent tumor control in a histologyindependent manner.13,24,27 However, this technique is limited when the tumor and spinal cord are in close proximity.
Finally, it is important to identify the tumor type, as certain histologies are particularly hypervascular. Metastases from renal cell, thyroid, hepatocellular, melanoma, and giant cell tumors can all bleed substantially during tumor resection. Preoperative embolization can be very effective in reducing intraoperative blood loss.28 One must identify hypervascular tumors preoperatively in order to take advantage of this procedure.
To confirm the histology in a patient with a spinal tumor, percutaneous biopsy may be required. This is not generally necessary when a patient with a known primary and active metastatic disease presents with a new spinal lesion. However, when there is no known primary or when a patient with a known primary has been without active disease for a prolonged interval, biopsy should be strongly considered in order to confirm the diagnosis, to exclude a second primary, or to rule out a primary bone tumor. This may be particularly true in patients who have a history of prior chemotherapy or radiation, which may increase the chance of a second malignancy.
Extent of Metastatic Disease/Systemic Staging
In addition to the type of tumor, the presence and extent of extraspinal metastatic disease have an impact on decision making. The presence of additional visceral and bone metastases adversely affects survival, which in turn may have an impact on the choice of treatment.21,22 This is the basis for the well-known Tomita scoring system, which assigns point values to these three factors to generate a score, which in turn determines the aggressiveness of treatment. Grade of malignancy (slow growth, 1 point; moderate growth, 2 points; rapid growth, 4 points), visceral metastases (no metastasis, 0 points; treatable, 2 points: untreatable, 4 points), and bone metastases (solitary or isolated, 1 point; multiple, 2 points) are used to generate a score from 2 to 10. A prognostic score of 2 to 3 points indicates a wide or marginal excision for long-term local control; 4 to 5 points indicates marginal or intralesional excision for middle-term local control; 6 to 7 points indicates palliative surgery for short-term palliation; and 8 to 10 points indicates nonoperative supportive care for end of life. Cancer therapies have evolved considerably since the publication of this paper. As a result the treatments recommended based on the prognostic score may no longer be optimal. Nonetheless, the impact of these prognostic factors on survival is clear. Patients with extensive systemic disease have a poorer survival and are less likely to benefit from major surgical procedures. Moreover, the increased disease burden may cause comorbidities (e.g., decreased pulmonary function from lung metastasis, coagulopathy from liver metastasis), that render the patient less able to tolerate larger procedures. Therefore, staging is mandatory and should be performed in all patients prior to surgery, if possible.
It is worth noting the aforementioned prognostic factors characterized by Tomita; tumor histology, visceral metastasis, and bone metastasis, are also components of the well-known Tokuhashi scoring system. In their system, Tokuhashi et al22 consider six key prognostic factors: general condition, number of extraspinal bone metastases, number of metastases in the vertebral body, presence or absence of metastases to major internal organs, site of the primary lesion, and severity of palsy. In addition to the primary site of the cancer (tumor histology), they evaluate presence of metastases to the major internal organs and score these as irremovable, removable, and none. They separate bone metastases into extraspinal and vertebral classifying the former as ≥ 3, 1 to 2, or 0, and the latter as ≥ 3, 2, or 1. Finally, they stratify the general condition of the patient (Karnovsky Performance Status) as poor (10–40), moderate (50–70), or good (80–100), and the presence of spinal cord palsy as complete, incomplete, or none. Each parameter is then assigned a range of scores to provide a maximum total of 15. The score is then used to determine how aggressive a treatment to select. Patients with lower scores are recommended for more conservative approaches, with higher scoring patients receiving excisional surgeries. The consistency rate between the criteria for predicted prognosis and the actual survival period was high in patients within each score range (0–8, 9–11, or 12–15), 86.4% in the 118 patients evaluated prospectively after 1998, and 82.5% in all 246 patients evaluated retrospectively. The prognostic criteria scoring system were useful for predicting the prognosis irrespective of treatment modality or local extension of the lesion.
Extent of Previous Treatment
The final component of the patient’s oncological status is the nature and extent of the prior therapy. This is not easy to stratify or quantify, and it needs to be evaluated on a patient-bypatient basis; however, the concept is relatively self-evident. Simply put, when considering treatment options for a patient with spinal metastatic disease, the choice will be influenced by what therapies have already been utilized and the relative efficacy of the remaining treatment strategies. As an illustrative example, a patient has metastatic breast cancer and a midthoracic lesion that is abutting the cord, causing some mild radiculopathy but no neurologic dysfunction. If this patient is therapy naïve, treatment options may include conventional radiotherapy, hormonal/chemotherapy (depending on receptor status), or surgery. But if this lesion has been previously irradiated and the patient is receiving thirdline chemotherapy, nonsurgical options may be limited, and surgery may be necessary if the prognosis is reasonable. The presence and proximity of prior radiation fields is often a determining factor regarding the efficacy or feasibility of subsequent radiation due to issues of spinal cord tolerance. Spinal SRS may help to minimize cord toxicity but requires that there be some degree of spatial separation between the tumor and the neurologic structures.24,29 Clearly, patients who have received multiple prior therapies may be further along in their overall disease trajectory. This may reflect a decreased overall prognosis that must be considered prior to surgery.17,18 Collaboration among the medical oncology, radiation oncology, and surgical teams is necessary to develop optimal treatment plans for these complicated patients.
 Feasibility of the Surgical Plan
Feasibility of the Surgical Plan
The final factor that must be considered before intervening surgically for a spinal metastasis is the feasibility of the surgical plan. The goal of treatment for spinal metastatic disease is palliation. Surgery must be able to reduce pain, restore and protect neurologic function, and restore spinal stability in a manner that is durable over the remaining life expectancy of the patient and with acceptable morbidity. Quality of life should be enhanced. There is ample evidence to suggest that surgery can help to achieve these goals, and in certain circumstances, particularly in the case of MESCC, may be the superior treatment option. In the case of high-grade MESCC, when combined with postoperative radiation, surgery provides far superior outcomes than does radiation alone.9,10,30 Moreover, surgery may be the only means of correcting symptomatic spinal instability. Lastly, surgery may be the best option in cases where chemotherapeutic and radiotherapeutic strategies have failed or are otherwise limited. However, surgery also tends to be among the most invasive treatments for spinal metastases and carries significant potential for complications. This is particularly relevant in a patient population that may have substantial comorbidities related to advanced age; underlying disease; prior treatment with chemotherapy, radiation therapy, or steroids; and poor nutritional status.7,31,32
In short, it is the responsibility of the spinal surgeon to carefully consider the surgical plan prior to taking a patient to the operating room. First, the surgeon must consider the strategy for resection of the metastasis. Will the resection be intralesional or en bloc? Given the palliative nature of surgery for metastatic disease, most resections are performed in a piecemeal fashion. However, there are some circumstances (indolent histology, solitary spinal metastasis, long predicted survival) where a more aggressive en bloc resection may be considered.33 In addition, for hypervascular histologies, a preoperative embolization should be considered to reduce intraoperative blood loss.28
Second, the surgeon must consider the surgical approach. Often this is a matter of individual preference; however, there are certainly regional anatomic constraints that may influence this decision, and these are discussed in a subsequent chapter.13,34 There may also be factors related to the individual patient that influence the choice of surgical approach. For example, the surgeon may wish to avoid operating in a previously radiated or operated site in order to reduce wound healing complications and make the dissection easier.31 Alternatively, it may not be feasible to consider a transthoracic approach in a patient with compromised pulmonary function. It is imperative for the surgeon to consider whether an access surgeon with additional expertise might provide a safer and more satisfactory surgical approach.
Third, the surgeon must consider the strategy for spinal reconstruction and stabilization. There are numerous devices, materials, and techniques that are available for rebuilding the spine following resection of a spinal metastasis. It is beyond the scope of this chapter to review this topic or the biomechanical principles of spinal reconstruction. However, there is one important point that the spine surgeon must contemplate in the spinal metastasis patient—the quality of the patient’s bone. The stability of a spinal reconstruction and stabilization relies on the implants contacting and fixating into bone of satisfactory quality. When this is not the case, implants can loosen and fixation can be compromised, leading to spinal instability. The presence of tumor in adjacent or nearby vertebra is one factor that can impact fixation. Imaging studies should be scrutinized to make sure that there is limited tumor burden in vertebra that is being relied on to provide adequate structural support. In addition, osteopenia and osteoporosis are common among cancer patients.35 This may be a by-product of advanced age, female sex, or treatments for the underlying cancer including steroids, chemotherapy, hormonal therapy, and radiotherapy. Poor nutritional status may also lead to bone loss. The spine surgeon must recognize this potential problem and avoid surgery, alter the reconstruction plan technique, or consider the adjunctive use of vertebral augmentation to improve the strength of the vertebra and fixation.
Lastly, the spine surgeon must consider wound healing. A palliative spine surgery that decompresses the neurologic elements and stabilizes the spine is not successful if the patient is left with a nonhealing wound. This can lead to a prolonged hospital stay and, more importantly, can delay the administration of muchneeded systemic therapy. It is incumbent upon the surgeon to recognize factors that will impede healing. These include prior radiation or surgery, chemotherapy, steroids, and malnutrition. Obviously, if previous radiation and surgical fields can be avoided, this is advantageous.31 However, this is often not the case. If the surgery is elective, there may be time to discontinue chemotherapy or steroids and improve the nutritional status of the patient. Unfortunately, most surgeries for spinal metastatic disease are done under more urgent circumstances. Therefore, the surgeon is frequently left with a situation that requires surgery, but presents wound healing challenges. In these situations, we strongly recommend collaboration with a plastic surgeon for immediate flap reconstruction at the time of the surgical resection and stabilization.36,37 The utilization of local muscle advancement flaps, rotational flaps, and even free tissue transfer at the time of the initial spine surgery can dramatically reduce complications related to wound healing.
Consideration of these aforementioned factors when planning surgery for spinal metastatic disease will help avoid poorly conceived operations, reduce complications, and lead to improved patient outcomes.
 Treatment Algorithms
Treatment Algorithms
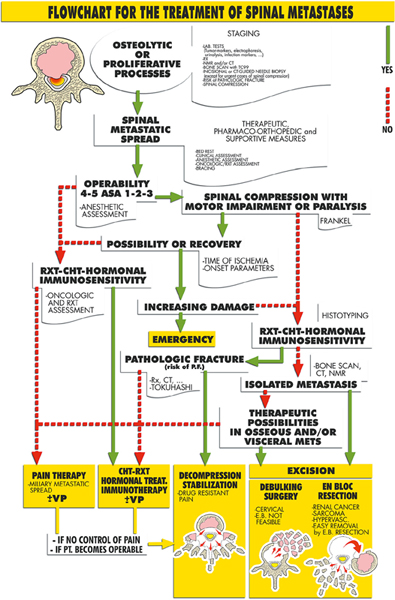
Utilizing the principles of evaluation and decision making outlined in this chapter, several authors and institutions have developed algorithms for the management of patients with spinal metastases. Obviously, treatment decisions must be made on an individual basis and not all patients will fit neatly within any procedural framework. Moreover, each institution will base its algorithm on the treatment modalities available within that center. Nonetheless, it is instructive to see how the key factors described above are integrated into two of the most commonly utilized treatment algorithms for patients with metastatic disease to the spine. These are the Algorithm for Spinal Metastases (Fig. 1.2) developed and prospectively applied by Boriani’s group in Bologna since January 2002,38–40 and the neurologic, oncological, mechanical, and systemic (NOMS) decision framework (Fig. 1.3) utilized during the past 15 years at Memorial Sloan-Kettering Cancer Center.17
In both treatment paradigms, a critical initial assessment is the overall medical status of the patient. In the Boriani algorithm, this is referred to as operability, and in the NOMS it is the systemic assessment. Those patients unable to tolerate surgery are referred for radiation or medical therapy. The next critical factor is the clinical presentation. In both frameworks the degree of neurologic compromise (measured either neurologically or by the degree of spinal cord compression) directs a patient toward surgery unless the histology is highly sensitive to chemotherapy or conventional radiation. Similarly, the presence of spinal instability (risk of pathological fracture in Boriani) leads the patient toward a surgical remedy. The oncological status of the patient is tightly intertwined with the clinical presentation. In particular, the impact of the tumor histology on response to radiation and chemotherapy is a critical factor. Chemoand radiosensitive histologies are more likely to be managed with nonsurgical modalities as long as there is no worsening neurologic compromise or spinal instability. Resistant histologies are directed toward surgery by Boriani’s algorithm. In NOMS, these tumors may be treated by spinal SRS (not available in Bologna) if the degree of cord compression is low grade. For high-grade compression with radioresistant histology, separation surgery to remove the compressive portion of the tumor and stabilize the spine, followed by radiosurgery to the remaining disease, is recommended.29 Implicit in both of these frameworks is that the surgeons carefully consider the feasibility of any treatment plan in advance of its execution.
Fig. 1.2 Boriani’s Treatment algorithm for spinal metastasis. ASA, American Society of Anesthesiologists; CHT, chemotherapy; CT, computed tomography; E.B., en bloc; Frankel, Frankel grading system; METS, metastases; NMR, nuclear magnetic resonance; PT., patient; RXT, radiation therapy; VP, vertbroplasty. (From Cappuccio M, Gasbarrini A, Van Urk P, Bandiera S, Boriani S. Spinal metastasis: a retrospective study validating the treatment algorithm. Eur Rev Med Pharmacol Sci 2008;12: 155–160. Reproduced with permission.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree