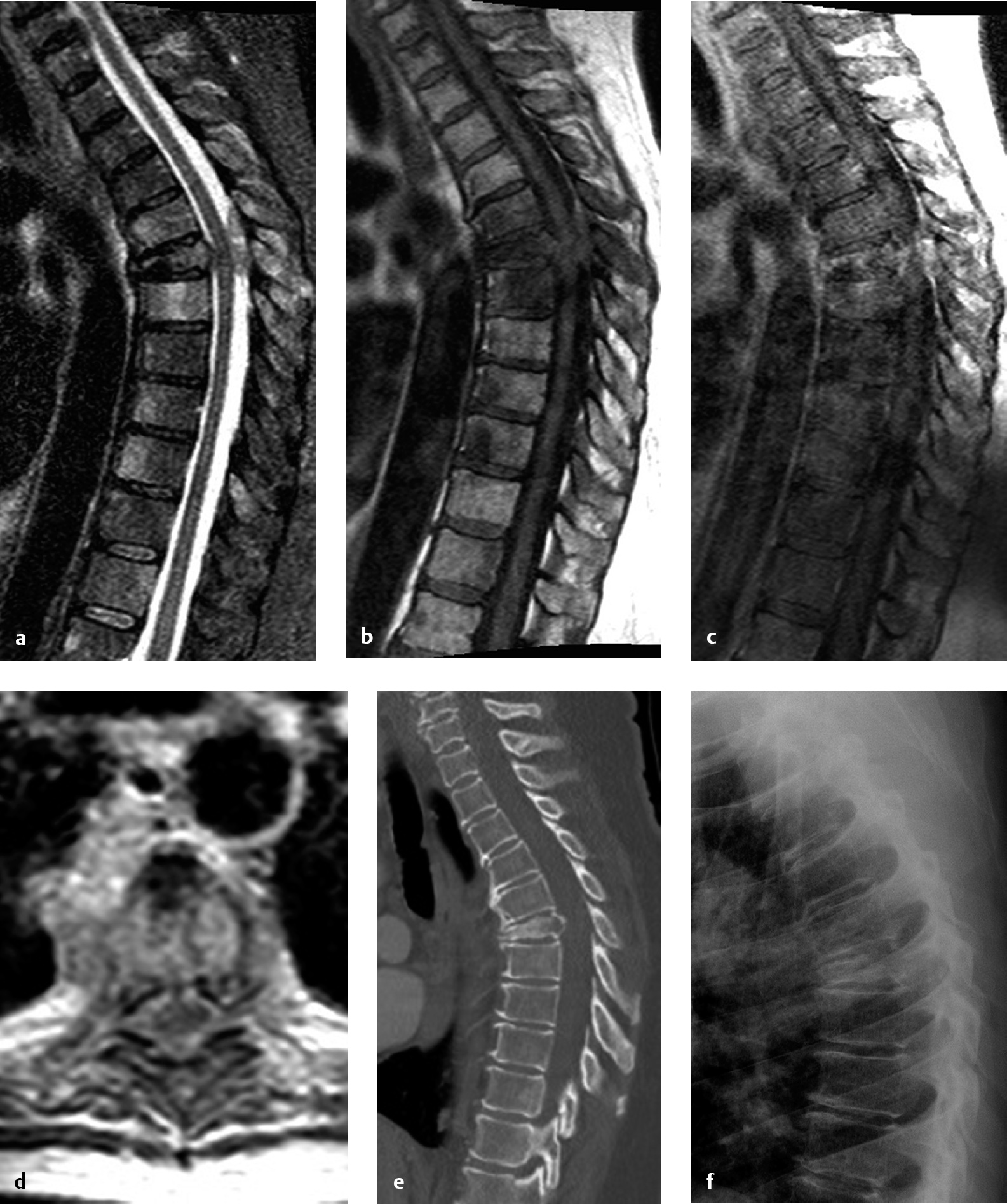
6 Surgery for metastatic spinal tumors was once a controversial topic, due to meager outcomes from decompressive laminectomy and unclear benefits compared to radiation alone.1–3 However, as surgical practice advanced to include metastatic tumor resection and spinal fixation/stabilization,4 neurologic outcome and pain results began to improve.1 The landmark 2005 Patchell et al5 study definitively changed management, establishing that circumferential surgical decompression plus adjuvant radiation is a favorable therapeutic strategy for patients with neurologic deficit from epidural spinal cord compression and an expected survival of at least 3 months. Strategies for spinal reconstruction and fixation have only grown in importance since, with around 25,000 new cases of metastatic epidural spinal cord compression yearly in the United States.6 After pulmonary and hepatic sites, the skeletal system is the next most common site of metastasis, and the spinal column is the most common skeletal site.6 When the pathological vertebral body is removed, the spinal column must be reconstructed in order to provide structural support. Reconstruction after resection of metastasis can generally be undertaken from an anterior, posterior, or combined approach. Techniques for vertebral body reconstruction vary widely, from the early use of polymethylmethacrylate with Steinmann pins7 or chest tubes8 to the more modern use of expandable titanium cages.9 Here we discuss clinical issues and preoperative planning, and summarize the key points and supporting data for each of these methods. Preoperative planning for spinal reconstruction cases is vital to ensure operative success. Planning includes acquiring adequate imaging, deciding whether to use an approach surgeon and whether to complete the procedure as a single stage or in multiple stages, considering preoperative tumor embolization, and scheduling the required equipment, including implanted hardware systems and neuromonitoring. Magnetic resonance imaging (MRI), computed tomography (CT), and plain film X-rays provide unique, complementary information in preparation for metastasis removal with spine reconstruction. As part of cancer staging, positron emission tomography (PET) or nuclear scintigraphy scans may often provide the first diagnosis of metastatic spinal cord compression, but generally they do not provide adequate resolution for preoperative planning.6 A contrast MRI scan helps to delineate the tumor boundaries and the degree of epidural spinal cord compression. MRI can clarify whether a metastatic tumor is confined to the vertebral body or extends to the posterior elements. CT best defines the bony anatomy; in addition to assessing the pathological bone invaded by tumor, the surgeon can assess bone quality at adjacent segments. Preoperative measurement of pedicle size and anatomy enables optimization of pedicle screw diameter, length, and trajectory. CT myelogram may be performed in patients with contraindications to MRI, such as implanted pacemakers or deep brain stimulators, but it carries the downside of being an invasive procedure with its own intrinsic risks. Standing or sitting spine X-rays demonstrate the effects of load-bearing, information unavailable from the standard supine spine MRI or CT scan. Sagittal and coronal balance should also be measured on weight-bearing X-rays (if possible), not on supine MRIs or CTs, in order to establish a preoperative baseline. Presurgical planning also involves deciding what type of intraoperative imaging to order. Anteroposterior (AP) and lateral C-arm fluoroscopy is used for localization in most cases. Intraoperative CT (when available) and navigation can be used for real-time image-guided implant placement or for ensuring proper placement of implants. It is sometimes beneficial to utilize an approach surgeon an approach surgeon who has expertise in anterior or lateral approaches. If anterior access to the upper cervical spine (C1-2) is required, an otolaryngologist–head and neck surgeon may be required for transoral approaches with or without mandible splitting. A thoracic surgeon is required for sternotomy for anterior access to the thoracic spine and may be beneficial for lateral thoracic approaches.10 A general or vascular surgeon may provide anterior retroperitoneal access to the lumbosacral spine. Even for spine surgeons who are trained in anterior and lateral approaches, it may be advantageous to arrange the services of an approach surgeon who has experience dealing with unexpected occurrences and maximizing patient safety. Region-specific approaches are discussed in greater detail in Chapter 5. For cases that involve expected long operative time or multiple anatomic approaches, some surgeons would consider staging in order to facilitate patient tolerance and to reduce surgeon fatigue. However, there is growing evidence to suggest that performing anterior and posterior fusions on separate days is not beneficial. A single-stage procedure may entail decreased overall operative time, lower blood loss, better deformity correction, and shorter hospital stay.11–13 In a recent study of the Nationwide Inpatient Sample, a 28% complication rate was observed with staged anterior-posterior surgeries, a significant increase over the 22% rate for same-day surgeries.12 With staging, there were increased rates of venous thrombosis and acute respiratory distress syndrome with no mortality benefit. Thoughtful consideration should be given to the option of preoperative angiogram with embolization in highly vascular lesions. A neurointerventional radiologist or endovascular neurosurgeon should be consulted for lesions deemed amenable to angiography or embolization. Angiography alone may be valuable to identify the segmental level and side of the artery of Adamkiewicz, especially when considering anterior or lateral approaches between T9 and L2.14,15 However, magnetic resonance (MR) or CT angiography should be considered as less invasive methods to obtain the same anatomic information. For certain hypervascular tumors, such as renal cell carcinoma, thyroid carcinoma, hepatocellular carcinoma, or hemangiopericytoma, preoperative embolization may minimize blood loss, enhancing safety and reducing operative time. However, embolization itself carries the risk of neurologic compromise. In one series, three of 12 patients (25%) suffered neurologic deficits after preoperative embolization.8 Therefore, risks and benefits must be carefully weighed prior to proceeding. Intraoperative neuromonitoring is commonly used for spine reconstruction with implants, although there is a paucity of data showing effect on neurologic outcomes. For cases with partial or no preoper ative motor or sensory deficit, motor evoked potentials (MEPs) and somatosensory evoked potentials (SSEPs) provide important feedback to the surgeon about whether any surgical manipulations nerve roots, albeit with the inherent risk of false negatives. An exception would be the case of complete spinal cord injury from metastatic spinal cord compression. Even in such cases, some clinicians would consider using preoperative MEPs and SSEPs to confirm a suspected complete spinal cord injury based on neurologic exam. For unstable spine lesions, preoperative supine MEPs and SSEPs are imperative to establish whether patient positioning causes any departure from baseline before the surgery commences. Additionally, direct stimulation of implanted screws intraoperatively may alert the surgeon to the presence of an unexpected breach from the intended trajectory. Anterior approaches have long been used for resection of vertebral body metastasis with reconstruction of the anterior column. An established technique in the thoracic spine entails anterior vertebrectomy, decompression, and reconstruction. The main benefits of an anterior approach are direct access to the diseased segment(s), improved wound healing, biomechanical strength from weight-bearing column reconstruction, and fixation of fewer segments.8 There are various options for vertebral body reconstruction supplemented with locking plate and screws. The anterior approach requires careful consideration of anatomic restraints and generally requires assistance of an approach surgeon. At T1-2, a combined anterior neck dissection and sternotomy is favored.8 At T3-4, an anterior neck dissection with partial sternotomy and anterolateral thoracotomy enables “trapdoor” entry to the chest cavity.8 T5-10 is less favorable for a pure anterior approach due to anatomic location of the heart, aortic arch, and great vessels; a right-sided thoracotomy is favored.6 At T11-L1, an anterior thoracotomy or anterior retroperitoneal approach may be necessary. Retroperitoneal approaches are generally used in the lumbar spine.16 Prior to commencing the vertebrectomy, segmental vessels should be identified, ligated, and transected. The intervertebral disks rostral and caudal to the pathological segments(s) should be removed with an annulotomy knife, rongeurs, and curettes. After removing cartilaginous layers, care should be taken to minimize the removal of subchondral cortical bone.9 Removal of end-plate bone increases the risk of graft subsidence. Vertebrectomy and removal of metastatic tissue then follows. The use of Leksell rongeurs at the outset enables the removal of larger pieces of diseased tissue and relative structural preservation in specimens sent to the pathology lab for diagnosis. A high-speed drill with a round or matchstick drill bit is necessary to remove posterior vertebral body tissue, particularly when nearing the posterior longitudinal ligament. An ultrasonic aspirator can be used to assist with tumor removal. The posterior longitudinal ligament is often opened to facilitate visualization of the underlying spinal cord dura and exiting nerve roots. A thorough decompression can only be ensured with direct visualization. Options for anterior column reconstruction include auto- or allograft bone, static and expandable cages, polyetheretherketone (PEEK) cages, and polymethylmethacrylate (PMMA) augmented with Steinmann pins. Prior to the widespread availability of mesh cages, PMMA was often used to fill in the vertebrectomy defect, historically combined with either a Steinmann pin or a chest tube as a scaffold. To accomplish the latter, a cylindrical defect was drilled into the superior and inferior vertebrae. A chest tube was cut to size and filled with PMMA.8 In an exothermic polymerization process, PMMA can heat surrounding tissues, so irrigation with lukewarm saline is beneficial.8 PMMA provides large surface area coverage of the end plate to minimize subsidence and is extremely stable and strong in compression. A more modern method of anterior column reconstruction entails the use of an expandable titanium cage.9 Expandable cages are increasingly employed due to their facility of use, restoration of vertebral height, and improvement in sagittal alignment and mechanical strength. Correction of sagittal alignment is a major advantage over methylmethacrylate, but higher cost is a disadvantage. After anterior column reconstruction, supporting instrumentation is implanted. An anterior locking plate with screws prevents distraction and stabilizes implants.8 In patients with poor bone quality or significant kyphosis, anterior fixation alone does not restore stability comparable to healthy segments in the thoracolumbar spine,17 and additional posterior supplementation should be considered in patients with extended life expectancy. When operating in the thoracic cavity, chest tubes are left in place during the early postoperative period. Although providing direct access, anterior thoracotomy approaches carry the highest complication rate: 39% (including 3.5% reoperation rate and 1.5% mortality); lateral extracavitary and costotransversectomy approaches carry lower morbidity rates of 17% and 15%, respectively.18 Despite the high complication rate, 76% of patients with neurologic compromise at presentation improved in some series.8 Costotransversectomy, transpedicular, and extracavitary approaches are viable options for posterior-only reconstruction in the lumbar19 and thoracic20 spine. They offer the benefit of anterior column reconstruction and posterior supplementation from a single approach. In addition, comorbidities or anatomic tumor burdens may preclude an anterior thoracic or abdominal approach and thus require a posterior-only approach. The three approaches differ in the degree of rib removal involved. The standard extracavitary approach requires removal of a substantial amount of rib with pleural dissection in order to access the spine. Costotransversectomies involve removal of the rib head in approaching the spine. Transpedicular corpectomies are performed entirely through the pedicle and do not require removal of the rib head nor pleural dissection. More commonly today these three approaches have been combined into one posterolateral approach; essentially whatever bone is needed to be removed to accomplish the surgical goals of decompression and reconstruction is removed. For placement of methylmethacrylate supplemented with Steinmann pins, a thin rim of cortical bone if often left as a mold.21 Modern techniques generally involve placement of an expandable titanium cage from the posterolateral direction. The previously used Luque rectangles and sublaminar cables have been replaced by pedicle screws as the standard posterior fixation. In the thoracic spine, lateral extracavitary approaches garnered greater interest prior to the era of transpedicular corpectomy.14 For these approaches, the patient is positioned prone or three-quarters prone. The lesion is localized with preoperative fluoroscopy. “Hockey-stick” (mid-line “handle” with lateral “blade”) or crescent-shaped incisions are surgical options. Skin and fascia are first opened at the midline. A lateral fascial incision at the injury level exposes the erector spinae, which are then elevated and retracted. The medial ribs, costotransverse joints, and costovertebral joints at the lesion level are dissected and cut/disarticulated. Removal of the rib requires careful dissection from the parietal pleura. Nerve roots are followed to the foramina. After removal of the ipsilateral pedicle, a vertebrectomy and diskectomy can be performed and the diseased level(s) addressed with the spinal cord under direct lateral visualization.14 An expandable cage or other graft (including the harvested rib segment) can then be placed to reconstruct the anterior column. Finally, pedicle screws and rods can be placed from the same approach due to dorsal exposure and access. Unfortunately, the lateral extracavitary approach carries a high morbidity of up to 55% in the thoracic spine and might be best reserved for lesions inaccessible from other routes. For transpedicular corpectomies, pedicle screw insertion can be completed via an open, mini-open, or percutaneous technique.22 In the open case, the fascia is fully opened, and paraspinal muscles fully dissected off the midline in the subperiosteal plane. They are retracted laterally to expose pedicle screw entry sites under direct visualization. For mini-open cases, skin is opened at the midline, but fascia is preserved. Pedicle screw placement then proceeds under either fluoroscopy or image-guidance with intraoperative O-arm CT scan. For fluoroscopy-guided pedicle screw placement, Jamshidi needles are placed into the lateral margins of the pedicles under AP fluoroscopy guidance, using the same technique as for percutaneous screw placement. They are then advanced about 10 mm under AP guidance, taking care not to violate the pedicle medially or inferiorly. A lateral view is taken to ensure correct trajectory at this point. Again under AP guidance, the Jamshidi is advanced to 20 mm, and a lateral image then confirms whether the needle has passed through the posterior cortex of the vertebral body. Blunt-tip Kirschner wires (K-wires) are inserted into the Jamshidi needles and advanced to a depth of approximately 75% of the vertebral body. The Jamshidi is then removed and a cannulated pedicle screw is advanced over the K-wire. Extreme caution and frequent imaging should be used to avoid inadvertent ventral migration of the K-wire. For image-guided pedicle screw placement, a single midline skin incision is made, again not opening the fascia. An intraoperative CT scan is performed with a reference marker on one of the caudal spinous processes. Then using intraoperative image navigation, a pilot hole is drilled with the navigated drill guide and drill. A navigated tap is used to prepare the entry site, and the appropriate screw size is measured. The pedicle screw is then advanced under navigation. Generally, three levels above and below the pathological vertebral body are instrumented, although two levels above and below can also be done in patients with excellent bone quality. It is critical to remember that rigid, durable fixation is essential for optimal pain relief and for preserving quality of life in patients with metastatic disease. Although pedicle screws can be placed in a mini-open or percutaneous fashion, transpedicular corpectomy requires a midline fascial opening. Paraspinal muscles are dissected and retracted laterally to the rib. After laminectomy, the pedicle is removed with a rongeur or drill. Vertebrectomy is then performed using rongeurs and drills until reaching the anterior longitudinal ligament. A matchstick drill bit is recommended in order to minimize the risk to ventral tissues. Complete removal of the vertebra is not always necessary and depends on the surgical goals. Before completing the corpectomy, a temporary rod should be placed on the contralateral side. A Woodson or down-angle curette is used to dissect the posterior vertebral body off of the spinal cord or thecal sac and advance it ventrally.21 The posterior vertebral body and posterior longitudinal ligament may contain tumor and should be removed. This is also necessary in order to achieve circumferential decompression.21 The ipsilateral nerve root is ligated and transected preganglionically to create an entry path for the cage. The posterior longitudinal ligament is dissected free and opened, and the ventral and caudal disks are removed. Then a pathway along the rib head must be opened. The rib head can be removed or opened in a trapdoor fashion. The latter option avoids pleural dissection and the rib head can temporarily be swung open to allow placement of the cage.23 A trial sizer should be placed to determine the largest possible safe cage placement. Finally, an expandable cage is passed into the corpectomy defect and expanded. The position is verified with an X-ray. Final rods and set screws are placed. In the open case, posterior arthrodesis is performed with auto- or allograft bone chips subjacent to the rod if life expectancy longer than 6 months is expected. An illustrative case is shown in Figs. 6.1, 6.2, 6.3, 6.4, 6.5, 6.6. Fig. 6.1a–f Preoperative imaging in a 58-year-old woman with metastatic melanoma causing epidural spinal cord compression. The patient had an inability to ambulate and profound neurologic deficit. (a) Sagittal T2 magnetic resonance imaging (MRI) shows T6 pathological fracture with epidural spinal cord compression. (b) Sagittal T1 precontrast scan. (c) Postgadolinium T1 sagittal MRI scan shows enhancement in the T6 and T7 vertebral bodies as well as a posterior T6-7 enhancing mass lesion contributing to epidural spinal cord compression. (d) Axial T1 postgadolinium MRI scan at T6 shows circumferential epidural spinal cord compression. (e) Sagittal CT T-spine and (f) upright lateral chest X-ray scan better demonstrate the pathological collapse and kyphosis at T6.
Spinal Reconstruction and Fixation/Fusion
 Introduction
Introduction
 Preoperative Planning
Preoperative Planning
Imaging Modalities
The Surgical Team
Staging
Angiography and Embolization
Neuromonitoring
 Approaches
Approaches
Anterior Approach
Posterior-Only Approaches
Lateral Extracavitary Approach
Transpedicular Corpectomy
Spinal Reconstruction and Fixation/Fusion
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree