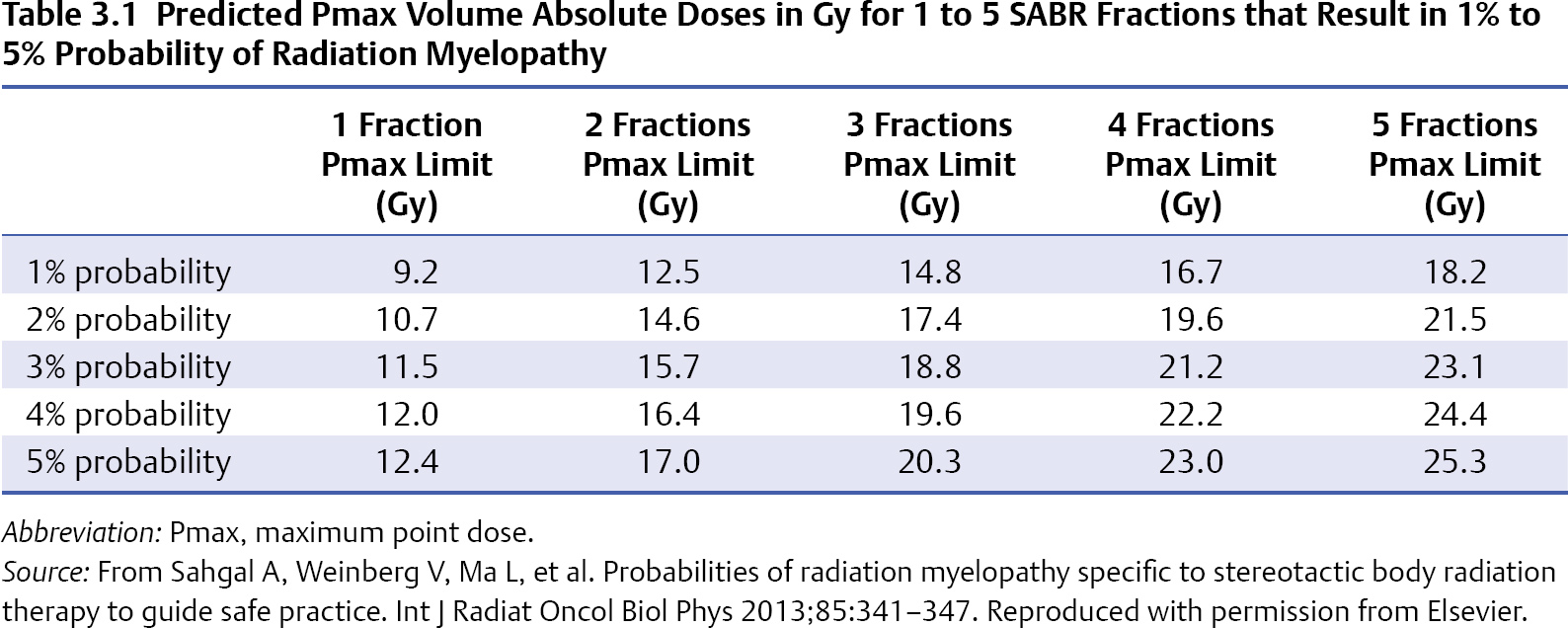
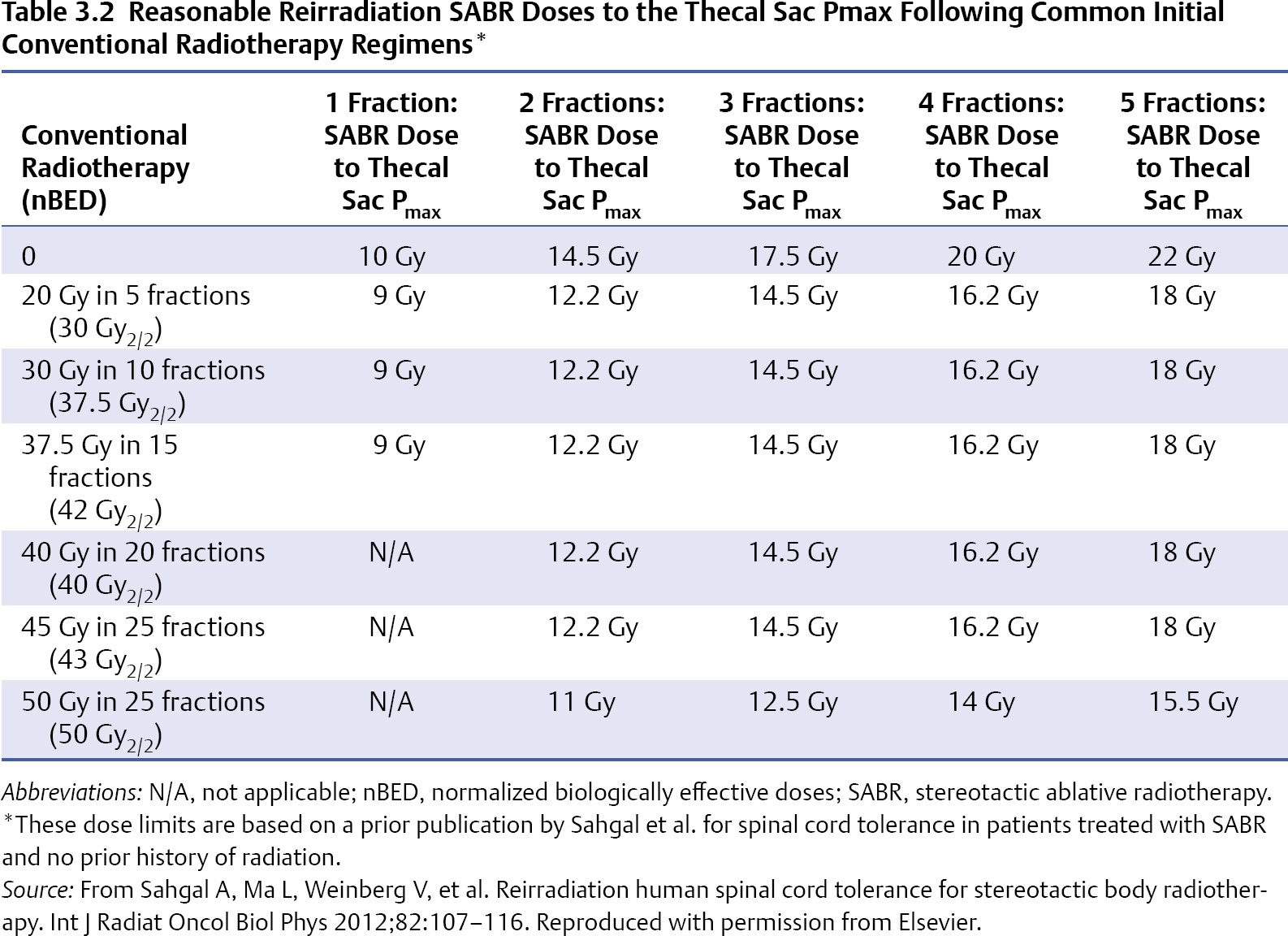
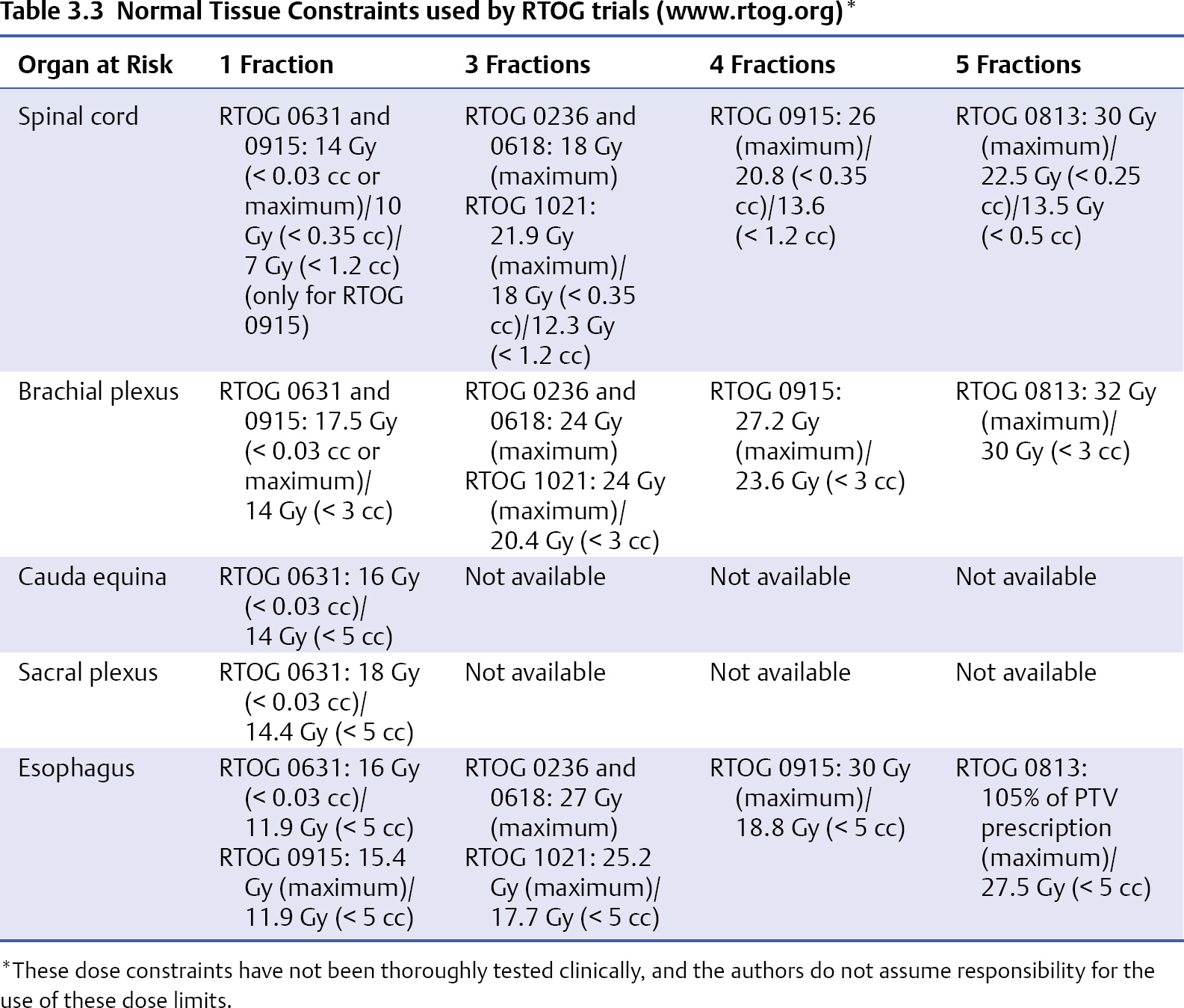
3 During the past decade stereotactic ablative radiotherapy (SABR), which is also known as stereotactic body radiotherapy (SBRT), has evolved as a high-dose targeted treatment aimed at local tumor control for various tumor sites such as the lung, the liver, and, now, the spine.1 With respect to spinal metastases, it was initially developed for the retreatment indication, as further conventional radiation was limited by the cumulative tolerance of the spinal cord, and spine SABR enables carving of the radiation dose around and away from the spinal cord to maintain safe dose limits while dose escalating the vertebral tumor segment. This technique has become a primary therapy for selected patients in the upfront setting and as postoperative therapy, and it is rapidly emerging as a commonly practiced alternative to low-dose conventional radiotherapy.2,3 Multiple series have shown promising results with regard to local control and pain control3; however, as yet there are no randomized controlled trials completed to confirm superior outcomes. Radiation Therapy Oncology Group (RTOG) 0631 is an ongoing trial comparing 8 Gy in one fraction delivered conventionally to 16 to 18 Gy in one fraction delivered with SABR. One of the major issues with this technique is the toxicity profile, which has not been extensively studied with phase 1 trials, and, moreover, there are limited prospective phase 2 trials. The fear is not so much acute toxicity but rather long-term toxicity, such as radiation myelopathy arising from overdosing the spinal cord, which is a delayed event and a devastating one for the patient, leading to paralysis, incontinence, and potentially death. This chapter summarizes the literature on adverse events with spine SABR, as several reviews have focused on clinical outcomes.2–4 Radiation myelopathy (RM) has been observed after spinal SABR in both unirradiated and reirradiated patients. The work by Sahgal et al5–7 at the University of Toronto has resulted in safe dose limits for both clinical situations based on the multi-institutional pooling of cases of RM and controls with no RM. For patients with no history of radiation, Sahgal et al7 recently reported on nine cases of RM after spinal SABR and provided an unprecedented detailed dose-volume histogram (DVH) analysis. The DVH data were compared for the nine RM patients and 66 controls, and the spinal cords were contoured based on the thecal sac as a surrogate for the true cord. This contouring approach was done to factor in potential sources of error in assuming that the dose to the contoured true cord is in reality the dose that is delivered. Sources of error include physiological spinal cord motion, intrafraction patient motion, variation in spinal cord delineation, potential errors in magnetic resonance imaging (MRI) and computed tomography (CT) image fusion, the treatment planning calculation algorithm, the image-guidance system, treatment couch motions, gantry rotation precision, and micro-multileaf collimator (mMLC) leaf position calibration. Basing the dose limits on the thecal sac essentially provides a margin of safety on the true cord that was anatomic as opposed to a fixed expansion. The authors acknowledge that this approach is roughly equivalent to a 1.5-mm margin expansion beyond the cord. All patients received SABR over one to five fractions, with fraction sizes ≥ 5 Gy. Although one patient with RM had been treated with boost SABR 6 weeks after external beam radiotherapy, the cumulative biologically effective dose (BED) was calculated. The median follow-up times for patients with and without RM was 23 and 15 months, respectively, and the median time to RM was 12 months (range, 3–15 months). In this study, all doses were converted to an equivalent BED in 2-Gy fractions, which was termed the normalized 2-Gy equivalent BED (nBED), and the α/β used for the spinal cord was 2 (hence, the dose unit is Gy2/2). This is also known as the equivalent dose in 2 Gy fractions (EQD2). Ultimately, a model was created to yield 1%, 2%, 3%, 4%, and 5% probabilities of RM based on linear regression analysis. The doses are listed in Table 3.1. The authors also reported a detailed analysis of the effect of dose within thecal sac volumes ranging from a point maximum (Pmax) to 2 cc. The most significant result was observed for the Pmax volume, corroborating the notion that the spinal cord is a serial organ. With respect to reirradiation, Sahgal et al6 also reported a case-control analysis based on five cases of RM and 16 controls. Once again, the thecal sac was used as a surrogate for the spinal cord. The cumulative nBED, which was defined as the sum of the nBED from the first course of conventional radiotherapy and the Pmax nBED from the SABR retreatment course for each patient was then calculated (using an α/β of 2 for the spinal cord). Based on the analysis, the authors recommended that the cumulative nBED to the thecal sac Pmax should not exceed 70 Gy2/2, which was most applicable when the initial conventional radiotherapy nBED ranged from 30 to 50 Gy2/2, and that the thecal sac (surrogate of spinal cord) Pmax nBED from reirradiation with SABR should not exceed 25 Gy2/2. In addition, the model suggested that the thecal sac SABR Pmax nBED/cumulative Pmax nBED ratio should not exceed 0.5, and the minimum time interval between the two treatment courses be at least 5 months. Based on the data of this study, Sahgal et al have made recommendations on absolute dose limit for the thecal sac for retreatment of spinal tumors with SABR (Table 3.2). Their colleagues at the University of Toronto follow these recommendations strictly in their high-volume centers and have not observed RM in their patients (unpublished information provided by Arjun Sahgal). Controversy The recommendations of Sahgal et al were based on BED modeling to convert the various dose-fractionation schemes into a single number, as it represents at this time the easiest model to apply in the clinic with the least number of assumptions. However, the linear-quadratic (LQ) model has recently been challenged in its ability to accurately estimate the BED in the ablative dose range (> 15 Gy/fraction) as used in SABR. Wang et al8 have proposed a generalized LQ (gLQ) model, which provides a natural extension across the entire dose range. This model was independently validated for tumor response in in-vitro studies at Thomas Jefferson University, but it had not been employed to model toxicities in normal tissues9 until Huang et al10 reanalyzed the data from the above discussed paper on spinal cord tolerance for reirradiation with SABR by Sahgal et al, using the gLQ model. It was also determined that no RM was observed when the cumulative Pmax nBED to the thecal sac was ≤ 70 Gy2/2 based on conversions using the gLQ model.10 However, with the scarcity of clinical data, the gLQ model must be approached with extreme caution, and clinical validation is necessary. Vertebrae bearing metastases are prone to pathological fractures due to the replacement of healthy bone with tumor. Lytic disease is inherently weaker than sclerotic disease; however, both increase the risk of skeleton-related events. Radiotherapy, frequently used as a treatment for bone metastasis, can increase the risk of fracture, but the risk is thought to be low with conventional palliative doses. However, SABR is based on ablative doses of radiation and delivered to the clinical target volume (CTV), which typically includes the entire vertebral body; hence, tumor and normal bone tissue are exposed. It is only recently that vertebral compression fracture (VCF) after spinal SABR has been reported in the literature in detail.11–13 Researchers at Memorial Sloan-Kettering Cancer Center (MSKCC) first reported their observation of VCF following SABR for spinal metastases. The dose regimen used was 18 to 24 Gy in one fraction, with a majority of patients receiving 24 Gy in one fraction. They observed progressive VCF in 39% of the vertebrae treated at a median time of 25 months.13 The location of spinal metastasis (above T10 vs T10 or below), the nature of the spinal metastasis (lytic vs sclerotic and mixed), and the percentage of vertebral body involvement were identified as predictors of VCF. Compared to the study from MSKCC, researchers at M.D. Anderson Cancer Center (MDACC)11 and the University of Toronto12 reported much lower rates of VCF and at an earlier time-course post-SABR (median time ranging from 2 to 3.3 months). In the MDACC study, the incidence of new or progressive VCF in 93 patients with 123 spinal metastases treated with SABR was 20%. Unlike the MSKCC study where a single-fraction regimen was used for SABR for all patients, approximately two thirds of the patients in the MDACC cohort received either 27 Gy in three fractions or 20 to 30 Gy in five fractions. Factors predicting development of VCF included age > 55 years, a preexisting fracture, and baseline pain. Obesity was found to have a protective effect. The median time to fracture progression was 3 months after SBRT. Similarly, the study from the University of Toronto, which included 90 patients with 167 spinal metastases treated with SABR, also included patients treated with two to five fractions in addition to those treated with a single fraction. The identified risk factors for VCF included the presence of kyphosis/scoliosis, lytic appearance, primary lung and hepatocellular carcinoma, and a dose per fraction ≥ 20 Gy. The crude rate of VCF was 11% and the 1-year fracture-free probability was 87.3%. The median time to fracture after SBRT was 2 months. The much higher rate of VCF observed in the MSKCC study was likely related to the very aggressive regimen of 24 Gy in one fraction used for the majority of the patients in that study.13 This corroborated with the finding from the University of Toronto study that a dose per fraction of ≥ 20 Gy was associated with an increased risk of VCF,12 which implies that the radiation has an independent effect on the risk of VCF. Moreover, a clinicopathological study from the University of Toronto found that radiation necrosis of the bone is likely the underlying mechanism of the fracture, which again makes radiobiological sense.14 However, there was a discrepancy of the time frame within which VCF occurred between the MSKCC study and the MDACC and University of Toronto studies. The much later median time to VCF reported by the MSKCC group at 25 months versus 3 and 2 months by the MDACC and University of Toronto11–13 may imply that with further follow-up the risk of VCF may continue to rise or that the pathological processes may be different. Pain flare, defined as a transient increase in pain during or shortly after radiotherapy, has been observed after conventional radiotherapy for bone metastasis. So far, there has been only one study addressing pain flare specific to spine SABR. Chiang et al15 at the University of Toronto performed a prospective study examining the incidence of pain flare and the predictive factors of the complication. A total of 41 steroid-naïve patients were enrolled, and pain was assessed using the Brief Pain Inventory (BPI) at baseline, during SABR, and for 10 days after SABR. The investigators recorded the use of pain medications with dosages converted to an oral morphine equivalent dose (OMED), and the use of steroids, daily during the study period. Pain flare was defined as In terms of SABR dose, 18 patients received 20 to 24 Gy in a single fraction and 23 patients received 24 to 35 Gy in two to five fractions to their spinal metastases. Pain flare was observed in 28 (68.3%) of 41 patients, with eight (28.6%) of 28 patients experiencing pain flare the day after SABR completion. Fifteen (53.6%) of 28 patients had a 2-point increase in worst pain score with no change in analgesic intake, five (17.9%) needed a 25% increase in analgesic intake with no decrease in worst pain score, and eight (28.6%) needed initiation of dexamethasone. With respect to risk factors associated with pain flare, none of the dosimetric or tumor-specific factors were predictive of pain flare. Only the Karnofsky Performance Scale (KPS) and the location of the index vertebra (cervical and lumbar) were predictive. Rescue dexamethasone at 4 mg orally once daily for the remainder of the course of SABR, or for 5 days after SABR either during or within 10 days from completion of SABR, was found to effectively treat the pain flare. The esophagus is located immediately in front of the spinal column, especially for the thoracic segments, and is susceptible to injury by ablative doses of radiation delivered to the spine. There is limited literature specific to esophageal toxicity from SABR. In a large study from MSKCC in which 182 patients with 204 spinal metastases abutting the esophagus were treated with single-fraction SBRT to a dose of 24 Gy, esophageal toxicity was scored according to the National Cancer Institute Common Toxicity Criteria for Adverse Events version 4.0.16 There were 31 (15%) acute and 24 (12%) late esophageal toxicities during the median follow-up interval of 12 months. Grade 3 or higher acute or late toxicities were observed in 14 (6.8%) patients. For grade 3 or higher esophageal toxicities, the median splits for D2.5cm3 (the minimum dose to the 2.5 cm3 receiving the highest dose), V12 (the volume receiving at least 12 Gy), V15, V20, and V22 were 14 Gy, 3.78 cc, 1.87 cc, 0.11 cc, and 0 cc, respectively. They also note the maximum point dose should be kept below 22 Gy. Importantly, the seven patients who developed grade 4 or higher toxicities either had radiation recall reactions after chemotherapy with doxorubicin or gemcitabine, or iatrogenic esophageal manipulation, such as biopsy, dilatation, and stent placement. In a study from Stanford University, 31 patients treated with SABR for lung or spinal tumors < 1 cm from the esophagus were reviewed to determine esophageal tolerance.17 Treatment regimens included 16 to 25 Gy in one fraction, 8 to 12 Gy × 2 fractions, 8 Gy × 3 fractions, 6 to 12.5 Gy × 4 fractions, and 5 to 10 Gy × 5 fractions. Three of 31 patients developed esophageal toxicities and two of them died of either a tracheoesophageal fistula or esophageal perforation (grade 5). Dosimetric parameters examined included D5cc, D2cc, D1cc, and Dmax. When the LQ model was used to convert the dose to a single fraction, the D5cc, D2cc, D1cc, and Dmax for the three patients ranged from 10.7 to 16.5 Gy, 13.7 to 18.2 Gy, 15.7 to 19 Gy, and 18.5 to 22.8 Gy, respectively. When the universal survival curve (USC) model was used to convert the dose to single fraction, the corresponding numbers were 11.9 to 16.5 Gy, 17.4 to 18.2 Gy, 19 to 22.5 Gy, and 21 to 37.3 Gy, respectively. Further data and modeling are required before hard dose limits are known, but both these studies suggest keeping the maximum dose ≤ 20 Gy. Given the close proximity of the spinal nerves and nerve plexuses to the vertebrae, these structures are susceptible to injury by ablative doses of radiation delivered through SABR. Although rare, radiation radiculopathy or plexopathy has been observed. In a phase I/II trial of single-dose SABR for radiation naïve spinal metastases from MDACC, where doses of 16 to 24 Gy were given, of 61 patients treated, 10 developed mild (grade 1 or 2) numbness and tingling and one developed grade 3 radiculopathy at L5.18 Researchers at Beth Israel Deaconess Hospital (Boston, MA) observed four cases of persistent or new radiculopathy in their cohort of 60 patients with recurrent epidural spinal metastases treated with SABR. All of those patients had radiological progression of disease, and it is unclear whether the complications were caused by tumor progression, radiation injury of the spinal nerves, or a combination of both.19 Investigators from MDACC observed two cases of grade 3 lumbar plexopathy in their study on reirradiation with SBRT for recurrent spinal metastases in 59 patients.20 The tolerance of the brachial plexus to SABR has been investigated in a study from Indiana University, where 36 patients with 37 apical primary lung cancers were treated with a dose of 30 to 72 Gy in three or four fractions.21 Seven cases of grade 2 to 4 brachial plexopathy was observed. The cutoff dose was determined to be 26 Gy in three or four fractions, which is in keeping with the constraint of 24 Gy in three fractions used in the RTOG trials. The 2-year rates of brachial plexopathy were 46% and 8% when the maximum brachial plexus dose was > 26 Gy and ≤ 26 Gy, respectively. One caveat of the study is that the subclavian/axillary vessels, which served as a surrogate for brachial plexus, instead of the full brachial plexus were contoured. To minimize the risks of serious complications caused by spinal SABR, all relevant organs at risk (OARs) such as the spinal cord, cauda equina, nerve plexuses and roots, and esophagus should be contoured and the dose constraint for each OAR should be respected. For critical neural structures such as the spinal cord and cauda equina, MRI is required for very accurate contouring.22 Fusion of the spinal MRI (axial T1 and T2 sequences) with the treatment planning CT should be performed to facilitate the process. The quality of the fusion should be carefully checked before the image sets are used for delineation of the neural structures. In patients who cannot undergo a spinal MRI or in the postoperative setting where there is significant metallic artifacts on MRI obscuring the visualization of the spinal cord, a CT myelogram can be used for delineation of the spinal cord. It is crucial that the window leveling is correct because inaccurate window leveling will lead to inaccurate cord contouring, which in turn will result in inaccurate determination of cord dose. Universal to SABR for all body sites and particularly for spinal tumors, robust immobilization is of the utmost importance. Most OARs including critical neural structures like the spinal cord and nerve roots are in very close proximity to the spinal CTVs, and the dose gradient between the spinal cord and spinal CTV is typically very steep in SABR for spinal metastases such that even slight deviations in positioning may result in overdosing of those structures, leading to catastrophic neurologic complications.5 When a linear-accelerator (LINAC)-based system is used for SABR, the use of the BodyFIX (Elekta, Stockholm, Sweden) near-rigid body immobilization system has been demonstrated by researchers at the University of Toronto to be more robust in minimizing intrafraction motions as compared to a simple vacuum cushion system, limiting the set-up error to 2 mm.23 Intrafraction patient motion occurs despite the application of advanced technology to its fullest extent, especially when the treatment time is anticipated to be long. Investigators from University of Toronto evaluated their LINAC-based system and showed that there could be intrafraction motion of 1.2 mm and 1 degree with near-rigid body immobilization (BodyFIX), image-guidance with kilovoltage cone-beam CT (CBCT), and a robotic couch capable of adjusting shifts with 6 degrees of freedom.24 To maintain this level of precision, it was concluded that intrafraction repeat cone-beam CT is necessary to check for any positional variation and at an interval of approximately every 20 minutes. Newer technologies like volumetric modulated arc therapy (VMAT) and the high dose rate flattening filter-free feature can drastically reduce treatment time and may render intra-fraction cone-beam CT unnecessary. With the use of a CyberKnife unit, which is capable of real-time intrafraction imaging with in-room stereoscopic kV x-ray and providing feedback to a mini-LINAC mounted on a robotic arm, a positioning accuracy of 1.0 mm and 1.0 degree can be achieved.25 Near-rigid body immobilization may not be necessary for this system as the CyberKnife is unique in its ability to reposition the LINAC while treatment is being delivered with tight tolerances. Apart from intrafraction patient motion, physiological spinal cord motion can also contribute to uncertainties or errors in the estimation of true spinal cord dose from SABR.5 Taking into account all the above-mentioned factors, it is prudent to create a Planning organ-at-risk volume (PRV) for the spinal cord to decrease the risk of RM caused by potential errors that can lead to overdosing of the spinal cord. Although some institutions and RTOG do not use a PRV to set a dose constraint for spinal cord, the authors routinely use the thecal sac or a 1.5- to 2.0-mm margin around the MRI/myelogram delineated cord to set the dose constraint for spinal cord. Sahgal et al6,7 have made recommendations on spinal cord dose constraints using the thecal sac as a surrogate in a radiation-naïve situation and a reirradiation setting (Tables 3.1 and 3.2). As mentioned above, one of the authors (Sahgal), who follows these recommendations strictly, has not observed any incident of RM, having treated at least 500 targets over the last 5 years. Alternatively, spinal cord dose constraints of 10 Gy in five fractions and 9 Gy in three fractions have been used by the other two authors (Lo and Chang) in the reirradiation setting, with prior conventional radiotherapy dose ≤ 45 Gy (1.8–2.0 Gy per fraction), and RM has not been observed. The reanalysis of the data on spinal cord tolerance for reirradiation with SABR using the gLQ model has yielded interesting results, which are different from those of the original study.10 However, extensive clinical validation is necessary to guide safe treatment. Currently, the data from the original study by Sahgal et al. represent the best clinical data available.6 As mentioned above, several risks factors have been identified, predicting VCF after SABR for spinal metastases. It seems to be prudent to avoid using a single fraction of ≥ 20 Gy,12 especially for patients with the above-mentioned risk factors. In patients with preexisting fractures, prophylactic kyphoplasty or vertebroplasty before SABR can be offered and may reduce the risk of further fracture and palliate the mechanical pain to enable the patient to tolerable subsequent SABR. For pain flare, rescue steroids are effective, and the use of prophylactic steroids may be best, subject to a further study at the University of Toronto. Medrol Pak Oral (Pfizer, Brooklyn, NY) is a commercially available prepacked version of methylprednisolone that is more convenient for patients to use during SABR. The esophagus is immediately anterior to the vertebral column mostly in the lower cervical and thoracic regions, and it is expected that some portions of the esophagus will receive a proportion of the dose delivered to the spinal metastasis. Therefore, it is crucial to contour the esophagus as an OAR and to respect its tolerance in order to minimize the risk of serious complications. Data from MSKCC showed that the maximum dose tolerated was volume-dependent, as mentioned above.16 Other factors that increase the risk of severe esophageal toxicity such as post-SABR doxorubicin- or gemcitabine-based chemotherapy or surgical esophageal manipulation should be avoided if possible. The use of a multiple session regimen (three to five fractions) may also decrease the risk of esophageal injury by SABR if the dose constraint cannot be met in one fraction. Nerve plexuses and nerve roots are susceptible to injury by ablative radiation.18–20 To spare these structures, they need to be carefully and accurately contoured. These structures can be better visualized on MRI, which can be fused with treatment planning CT. Best efforts must be made to respect the dose constraints of these neural structures, especially at levels where the nerves roots or nerve plexuses are responsible for motor function of the extremities. The contouring atlas of brachial plexus is available at the RTOG website (http://www.rtog.org/CoreLab/ContouringAtlases/BrachialPlexus-ContouringAtlas.aspx). Table 3.3 lists the constraints used by RTOG trials for the spinal cord, esophagus, cauda equina, and nerve plexuses. Note that the constraints in the table have not been tested clinically, and their use outside of a clinical trial setting is not authorized by RTOG. The major complications that have been observed after SABR for spinal metastases include RM, VCF, pain flare, esophageal toxicity, and nerve injury.6,7,11–20 Given the proximity of those relevant OARs to the vertebrae, these complications are not unexpected. Every effort must be made to spare those structures from doses beyond their tolerance. Robust near-rigid immobilization and strategies to manage intra-fraction patient motion are crucial steps in safe delivery of SABR. Apart from near-rigid immobilization, intrafraction CBCT and the use of newer technologies such as VMAT and the high dose rate flattening filter-free mode to drastically reduce treatment time can also tackle intrafraction patient motion.
Major Complications Associated with Stereotactic Ablative Radiotherapy for Spinal Metastasis
 Introduction
Introduction
 Major Adverse Events
Major Adverse Events
Radiation Myelopathy
Vertebral Compression Fracture
Pain Flare
Esophageal Toxicity
Radiation Plexopathy/Radiculopathy
Avoiding Complications
 Chapter Summary
Chapter Summary
< div class='tao-gold-member'>
Major Complications Associated with Stereotactic Ablative Radiotherapy for Spinal Metastasis
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree