Etiology of Hip Osteoarthritis
David W. Anderson
Harry E. Rubash
Introduction
Osteoarthritis is a common joint disorder also known as degenerative joint disease or degenerative arthritis. In osteoarthritis of the hip, the entire joint structure and function is affected because of degeneration of the tissues of the hip, including the hyaline cartilage, fibrocartilage, synovium, and bone. Degenerative joint disease of the hip represents a heterogeneous group of conditions that result in common radiologic and histopathologic findings. Attempts to delineate the etiology of hip osteoarthritis require an understanding of the differences between primary and secondary osteoarthritis, as well as an ability to separate these entities from other hip conditions.
The main symptoms of osteoarthritis are joint pain and stiffness. The pain is generally related to activity, may be worse toward the end of the day, and may also be present after periods of inactivity. Pain is usually felt in the anterior groin, but younger patients and patients with osteoarthritis because of dysplasia or femoroacetabular impingement may have pain present mainly with provocative maneuvers at the extremes of motion. There may be sharp pains present related to particular motions. Stiffness in the morning or after a period of inactivity is usually present, but generally lasts less than 30 to 60 minutes, which may help distinguish osteoarthritis from other arthropathies. The American College of Rheumatology has developed a classification criterion to distinguish osteoarthritis from other rheumatic diseases of the hip (1) (Table 8.1). This classification system is intended to utilize a group of clinical and laboratory data, with or without the inclusion of radiographs, to identify patients with osteoarthritis, and to distinguish osteoarthritis from other diseases. Clinical criteria without radiographic assessment are reasonably sensitive (86% sensitivity), but not very specific (75% specificity), for the diagnosis of osteoarthritis of the hip. The addition of a radiographic classification criteria increases sensitivity to 91% and specificity to 89% (1). The strength of the combined clinical and radiographic assessment in distinguishing osteoarthritis of the hip from other disease entities is the inclusion of radiographic findings of femoral and/or acetabular osteophytes. Many hip conditions cause axial joint space narrowing on radiographs; it is the combined finding of osteophytes and joint space narrowing which increases the proper definition of osteoarthritis in this classification system.
The etiology of osteoarthritis of the hip can be considered in the general categories of primary osteoarthritis and secondary osteoarthritis. The definition of primary osteoarthritis is vague and generally considered to be related to the aging process and wear on the joint over time. Broadly speaking, primary osteoarthritis is a diagnosis of exclusion and is also termed idiopathic, because of the inability to specifically define an underlying anatomic abnormality or specific disease process leading to the degenerative process. Secondary osteoarthritis results from conditions that change the environment of the cartilage, including trauma, congenital or developmental joint abnormalities, metabolic defects, infection, endocrine disease, neuropathic conditions, and disorders that affect the normal structure and function of the hyaline cartilage. Secondary osteoarthritis of the hip therefore occurs when a condition results in an anatomic abnormality, which can be relatively subtle, predisposing the hip to mechanical factors that lead to degenerative changes.
Table 8.1 American College of Rheumatology Classification Criteria for Osteoarthritis of the Hip | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||
The etiology of hip osteoarthritis has been the subject of great interest for many decades. Much of our understanding of osteoarthritis epidemiology began with cross-sectional population-based studies by Kellgren and Lawrence in Britain during the 1950s (2). The evaluations in this study were based upon single radiographs of the hand, spine, hips, knees, and feet. In 1953, Harrison published a paper describing the study of the nature and evolution of the disease (3). This research was an extensive investigation based on postmortem examinations of hip specimens ranging from normal hips to those with severe osteoarthritis (91 specimens; age range: 0 to 100), with many specimens having serial radiographs available to review as their disease had progressed over time. Harris, in 1986, described the etiology of secondary arthritis of the hip in great detail after reviewing roentgenograms of patients with so-called idiopathic osteoarthritis (4). This study came to the conclusion that there is a characteristic femoral head and neck shape associated with an increased incidence of degenerative joint disease of the hip. In addition, it was noted that prior work found a high prevalence of unrecognized acetabular dysplasia in patients with previously diagnosed idiopathic osteoarthritis of the hip. The last few decades have seen a resurgence of cohort-based epidemiologic studies using modern information technology to produce longitudinal information to help determine assumptions about causation. This allows identification of risk factors that are present preceding disease occurrence, assisting in endeavors to treat the early disease process. The efforts of these, and many other researchers, have contributed greatly to our current understanding of the multifactorial causes of hip osteoarthritis, from the mechanical process of femoroacetabular impingement to the cellular and molecular levels of individual chondrocyte necrosis, leading to the degeneration of the hip joint and clinical outcomes associated with the natural history of hip osteoarthritis.
Prevalence of Hip Osteoarthritis
Osteoarthritis is the most common rheumatologic condition, and the most frequent cause of musculoskeletal disability in developed countries. As the most common type of arthritis, it accounts for more disability and dependency in walking, stair climbing, rising from a seated position, and other lower extremity tasks than any other arthritic condition. In the general adult population, this disease has a profound impact on the activities of daily living, with the impact greatly affecting the elderly. Osteoarthritis can affect any joint in the body; however, it commonly affects the knee and hip joints. The effects of osteoarthritis on the large joints of the lower extremities result in reduced mobility, loss of independence, and increased utilization of health care services. It is essential to understand the basic epidemiologic features of osteoarthritis to examine possible associations between various intrinsic and extrinsic factors which cause the clinical symptoms and subsequent disability leading a patient to seek treatment.
The prevalence and incidence of osteoarthritis are directly correlated with age in multiple studies, as expected. In population surveys of United States adults published in 2008, it is estimated that nearly 27 million people have clinical osteoarthritis (5). Previous United States population-based studies have given good prevalence data. These include the National Health and Nutrition Examination Surveys (NHANES I, NHANES II, and NHANES III), the Framingham Osteoarthritis Study, and the Johnston County Osteoarthritis Project. These studies show that there is a higher prevalence of osteoarthritis in most joints comparing women to men, and women often have more generalized osteoarthritis than men of the same age (Tables 8.2 and 8.3). Before 50 years of age, men have a higher prevalence and incidence of general osteoarthritis than women. After age 50, women have a higher incidence and prevalence of general osteoarthritis.
These studies show that the sex difference in prevalence then increases slightly with age.
These studies show that the sex difference in prevalence then increases slightly with age.
Table 8.2 Prevalence of Radiographic OA in the Hands, Knees, and Hips, by Age and Sex, from Population-based Studiesa | ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||
Table 8.3 Prevalence of Symptomatic OA (Symptoms and Radiographic Changes of OA in the Symptomatic Joint) in the Hands, Knees, and Hips, by Age and Sex, from Population-based Studiesa | ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||
Multiple longitudinal and cross-sectional studies looking at racial and national differences have provided insights into the disease etiology. Still, there is conflicting evidence as to whether different ethnic groups are more susceptible to osteoarthritis even though studies show different rates of osteoarthritis in some populations. There is disconcordance among the various large population-based studies discussed below, leaving uncertainly regarding the true prevalence of hip osteoarthritis. The Johnston County Osteoarthritis Project was a study of hip and knee osteoarthritis in 2,997 African American and white individuals older than 45 years of age in a rural county in North Carolina. Comparing both ethnic groups, the overall prevalence of hip osteoarthritis was 27.6% in this study for radiographic hip osteoarthritis (6). In this study, Caucasians had a lower radiographic hip osteoarthritis (26.6%) and symptomatic hip osteoarthritis (9.2%) compared with African Americans (32.1% radiographic osteoarthritis and 12.0% symptomatic osteoarthritis). In another US community-based study of 4,855 Caucasian women older than 65 years of age, the overall prevalence was found to be only 7.2% for mild and 4.7% for moderate-to-severe radiographic evidence of osteoarthritis (7). A cross-sectional study in Greece from 2006 with a sample size of 8,740 patients partitioned patients into urban, suburban, and rural classifications. This study found an age- and sex-adjusted prevalence of hip osteoarthritis to be 0.9% in their total target adult population across all demographics (8). Sociodemographic factors on the prevalence of major joint osteoarthritis in this study are listed in Table 8.4. In this study, significant risk factors for hip osteoarthritis included being female, age greater than 50 years, and obesity. The authors offered a possible explanation for the variation in the prevalence of symptomatic knee, hand, and hip osteoarthritis among the three groups as differences in genetic factors, physical activities, lifestyle factors, and the prevalence of obesity. In this population, obesity defined as a body mass
index >30 kg/m2 had a 2.3 increased odds ratio for symptomatic hip osteoarthritis with a confidence interval of 95%. Obesity is a known risk factor for both hip and knee osteoarthritis (9,10). In this study, differences in the prevalence of symptomatic knee and hip osteoarthritis may be due, in part, to difference in the prevalence of obesity. In the Beijing Osteoarthritis Study (11), the overall prevalence of radiographic hip osteoarthritis in Chinese ages 60 to 89 years was 0.9% in women and 1.1% in men; it did not increase with age. Chinese women had a lower age-standardized prevalence of radiographic hip osteoarthritis compared with Caucasian women in the Study of Osteoporotic Fractures (7) and the National Health and Nutrition Examination Survey (12). Chinese men had a lower prevalence of radiographic hip osteoarthritis compared with white men of the same age. In the United States, the rate of hip osteoarthritis in people of Asian heritage is lower than that of Caucasians (13), suggesting a genetic or lifestyle-derived protective mechanism. In most studies, the incidence and prevalence of osteoarthritis appear to level off or decline in both sexes around 80 years of age. The reasons for this decline at age 80 are somewhat unclear and merit further investigation as to possible protective mechanisms against osteoarthritis in demographic.
index >30 kg/m2 had a 2.3 increased odds ratio for symptomatic hip osteoarthritis with a confidence interval of 95%. Obesity is a known risk factor for both hip and knee osteoarthritis (9,10). In this study, differences in the prevalence of symptomatic knee and hip osteoarthritis may be due, in part, to difference in the prevalence of obesity. In the Beijing Osteoarthritis Study (11), the overall prevalence of radiographic hip osteoarthritis in Chinese ages 60 to 89 years was 0.9% in women and 1.1% in men; it did not increase with age. Chinese women had a lower age-standardized prevalence of radiographic hip osteoarthritis compared with Caucasian women in the Study of Osteoporotic Fractures (7) and the National Health and Nutrition Examination Survey (12). Chinese men had a lower prevalence of radiographic hip osteoarthritis compared with white men of the same age. In the United States, the rate of hip osteoarthritis in people of Asian heritage is lower than that of Caucasians (13), suggesting a genetic or lifestyle-derived protective mechanism. In most studies, the incidence and prevalence of osteoarthritis appear to level off or decline in both sexes around 80 years of age. The reasons for this decline at age 80 are somewhat unclear and merit further investigation as to possible protective mechanisms against osteoarthritis in demographic.
Table 8.4 Estimated Adjusted Effects of Sociodemographic Factors on the Prevalence of Symptomatic Knee, Hand, and Hip Osteoarthritis in the Target Adult Population | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Sex- and age-related patterns found in osteoarthritis are consistent with the role of postmenopausal estrogen deficiency and some studies have proposed an increased risk of developing osteoarthritis with this mechanism. There is a known higher incidence of osteoarthritis in women who are over the age of 50. This is the approximate age of menopause, and estrogen loss has long been strongly implicated as a risk factor. Several epidemiologic studies show a reduction in the risk of hip and knee osteoarthritis with the use of estrogen replacement therapy (14,15,16,17,18). Other studies have found no significant effect of hormone replacement therapy on the incidence of osteoarthritis progression or joint pain (19,20,21). One study found an increased risk of clinical osteoarthritis in community-dwelling postmenopausal women aged 43 to 97 years who had used estrogen supplementation for at least 1 year after menopause (22). These competing data collectively suggest the possible protective effect hormone therapy on joint health; however, the exact mechanism or degree of risk reduction is still debated. Interestingly, human articular chondrocytes possess functional estrogen receptors, and there is evidence that their presence upregulates the synthesis of proteoglycans in vivo (23,24,25). The relationship of these molecular findings are currently under investigation.
There is not a single risk factor that alone is likely to cause osteoarthritis. Although considered multifactorial in many cases, heredity is believed to play an important role in the development of this disease process. Genetic studies hold the promise to help identify patients prior to the onset of functionally limiting disease and concentrate on modifiable risk factors. When risk factors are consistently identified in valid epidemiologic studies, inferences can be made to these factors being identified as a potential cause of the disease. These risk factors can also be used to describe the distribution, development, or progression of the disease in a particular population. Recent studies have focused on patient lifestyle, environmental risk, and racial differences.
Most population-based studies make a distinction between symptomatic osteoarthritis and radiographic osteoarthritis. Clinical or symptomatic osteoarthritis consists of joint symptoms including pain, aching, or stiffness associated with structural evidence of change, usually through radiographs. Patients can experience symptoms related to joint changes in varying degrees. In the United States, some studies have examined differences in self-reported osteoarthritis. These studies are limited by potential inaccuracies in the self-reporting tools and patient bias. Although they provide valuable contributions based on the large cohorts they involve, there is no valid objective evidence established in this information. Radiologic assessment of osteoarthritis allows objective evidence with criteria to assess cartilage loss, osteophytes, joint space narrowing, subchondral sclerosis, and cyst formation. This approach gives improved objective evidence compared to stratifying symptoms based on patient history or self-reported diagnoses. In addition, the Kellgren and Lawrence scale for grading osteoarthritis (26) can be applied when reviewing a radiograph, which allows improved standardization across various studies (Table 8.5). This system evaluates the severity of osteoarthritis based on the absence or presence of osteophytes, marked narrowing of joint space, severe sclerosis, and definite deformity of bone ends.
Table 8.5 Kellgren and Lawrence Radiologic Assessment of Osteoarthrosis (148) | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
Theories on the Pathogenesis of Osteoarthritis
The development of clinical and radiographic osteoarthritis is affected by both systemic and local host factors. Systemic
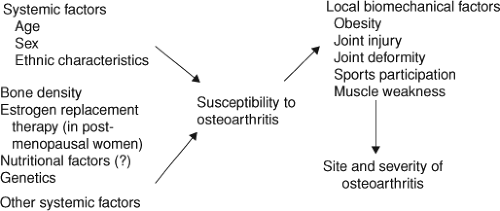
factors include a person’s age, sex, genetic susceptibility to cartilage vulnerability, and other possible unidentified factors. These systemic factors are part of the host environment which makes the cartilage less capable of healing damage caused by daily injuries. There are multiple mechanisms which are proposed to explain this process, including the effect of various growth factors and cytokines on chondrocytes and the synthesis of the cartilage matrix in the joint. Recent advances in cellular and molecular biochemistry have identified other systemic factors that might accelerate enzymatic destruction of the matrix and will be discussed further. In addition, there are possible local bone characteristics which have been identified as being protective or injurious to the cartilage and its ability to self-repair. A scheme of the pathogenesis of osteoarthritis and risk factors is presented in Figure 8.1 (27).
factors include a person’s age, sex, genetic susceptibility to cartilage vulnerability, and other possible unidentified factors. These systemic factors are part of the host environment which makes the cartilage less capable of healing damage caused by daily injuries. There are multiple mechanisms which are proposed to explain this process, including the effect of various growth factors and cytokines on chondrocytes and the synthesis of the cartilage matrix in the joint. Recent advances in cellular and molecular biochemistry have identified other systemic factors that might accelerate enzymatic destruction of the matrix and will be discussed further. In addition, there are possible local bone characteristics which have been identified as being protective or injurious to the cartilage and its ability to self-repair. A scheme of the pathogenesis of osteoarthritis and risk factors is presented in Figure 8.1 (27).
There are several biologic changes in the hip joint which could account for the increase in incidence and prevalence of osteoarthritis that occurs with the aging process. This includes a decreased responsiveness of chondrocytes to the reparative stimuli of various growth factors and intrinsic cellular mechanisms. The aging hip joint also experiences a relative laxity of ligaments around the joint, allowing micromotion which may potentiate subtle morphologic abnormalities and allow progression of previous asymptomatic degeneration. In addition, there is a slowing of peripheral neurologic response, which can allow alteration of the gait cycle and produce more stress through the affected joint. In summary, there is interplay between mechanical and biologic mechanisms which uncouples the normal maintenance, degradation, and synthesis of articular cartilage and extracellular matrix. This, in turn, is manifested by softening, fibrillation, ulceration, loss of articular cartilage, subchondral sclerosis, and eburnation of subchondral bone. As the disease progresses, osteophytes and subchondral cysts form, causing further alteration of joint mechanics and increased stress loading through the joint with the resulting clinical presentation of osteoarthritis.
A model for primary osteoarthritis is the premature degeneration of the joint due to premature chondrocyte senescence. This condition of the chondrocyte cells makes them more susceptible to osteoarthritis cartilage degeneration. Stressful conditions could push the tissue into an apoptotic state, or accelerate already fragile cellular conditions, allowing continuation of early disease processes. In this model, it is the cartilage matrix itself which is the initiating factor in osteoarthritis. Multiple studies have shown a genetic link to osteoarthritis susceptibility (28,29,30). Familial OA with classic clinical and radiographic findings is linked to the COL2A1 gene (30). COL2A1 is of particular interest for gene studies because it encodes type II collagen, the major structural collagen in cartilage, and mutations in COL2A1 have been found in several severe osteochondrodysplasias. There is disagreement in various studies as to the actual involvement of this gene in osteoarthritis not associated with severe osteochondrodysplasias. Evidence for this gene’s involvement include the wide spectrum of osteoarthritis phenotypes found in patients with mild chondrodysplasia, mild spondyloepiphyseal dysplasia, achondrogenesis, and hypochondrogenesis, which all involve the COL2A1 gene (31). Other studies using linkage analyses and direct sequencing looking for single nucleotide polymorphism (SNP) of the COL2A1 gene in familial generational studies do not show that COL2A1 is a susceptibility gene for early-onset osteoarthritis (32). There is considerably variable penetrance with these genes and consideration must be given to the population and additional factors which contribute to the presentation of clinical and radiographic osteoarthritis. Other candidate genes with contributions to the development of osteoarthritis are discussed in detail later in this chapter.
Mechanisms for the Development of Hip Osteoarthritis
In theory, joint cartilage can be damaged by either a single sudden major injury or through repetitive activities that exceed the ability of the joint and periarticular tissues to withstand the additive insults of multiple, smaller injuries. In animal studies, a severe injury followed by weight-bearing causes development of posttraumatic osteoarthritis in nearly all cases (33,34,35,36,37,38,39,40,41). This may be due to the profound impact and cellular death that occurs with the initial trauma or due to a change in biomechanics associated with the injury and progressive and early disease over a short time period. This change can include altered gait patterns, increased shear stress, or other forces on the hip joint. Recently, femoroacetabular impingement has been recognized as a cause of early osteoarthritis. Excessive contact stress can occur because of subtle developmental abnormalities, leading to early-onset osteoarthritis. The most frequent location for femoroacetabular impingement is the anterosuperior rim of the acetabulum (42). The pattern of damage to the acetabular cartilage depends upon the shape of the hip. Initially, a separation of the acetabular cartilage from the labrum is observed, followed by progressive degenerative changes if the mechanical insult is not addressed. Current radiographic analysis of hips presenting with femoroacetabular impingement shows a broad variety of hip morphology leading to the clinical presentation of hip pain and early degenerative disease. Dynamic examination of hips with femoroacetabular impingement has helped elucidate the biomechanical process leading to the progression of this degenerative process in young patients (43,44).
Femoroacetabular impingement first began to be recognized as a possible precursor to hip osteoarthritis nearly
50 years ago, when Murray described the “tilt deformity” of the hip (45) (Fig. 8.2). The deformity was poorly characterized by today’s standards, as only anteroposterior radiographs were available. Historically, descriptions of the morphology of the femoral head can be traced back into the late 19th century, when Charles compared the lower extremity osseous morphology between different ethnic groups in relation to function, finding differences in the articular area of the femoral head and neck, similar to what is described as asphericity in modern literature (46). Much more recent studies have shown there are improved methods to detect femoral head and neck asphericity, whereas anteroposterior and externally rotated cross-table radiographic views alone are likely to miss this “tilt deformity” (47,48,49,50). Harris (4) was able to expand on this idea with description of mild acetabular dysplasia and pistol grip deformities associated with early osteoarthritis of the hip. Ito et al. (50) coined the term “cam impingement” to describe the consequence of an aspherical junction between the femoral head and neck or an increased radius of the femoral epiphysis as it joints the neck. This was described using an MRI-based anatomical study of the femoral head–neck offset after observing damage to the labrum due to impingement in nondysplastic hips. Patients in the affected group showed a significant reduction in mean femoral anteversion and mean head–neck offset on the anterior portion of the femoral neck, correlating with the site of symptomatic impingement seen on the adjacent rim of the acetabulum during flexion and internal rotation of the hip. Gross abnormalities of the hip are well recognized as precursors to early hip degeneration; however, it was the recognition and recent advances in understanding the more subtle forms of hip abnormalities which are leading to earlier treatment modalities to possibly halt the progression of hip osteoarthritis today.
50 years ago, when Murray described the “tilt deformity” of the hip (45) (Fig. 8.2). The deformity was poorly characterized by today’s standards, as only anteroposterior radiographs were available. Historically, descriptions of the morphology of the femoral head can be traced back into the late 19th century, when Charles compared the lower extremity osseous morphology between different ethnic groups in relation to function, finding differences in the articular area of the femoral head and neck, similar to what is described as asphericity in modern literature (46). Much more recent studies have shown there are improved methods to detect femoral head and neck asphericity, whereas anteroposterior and externally rotated cross-table radiographic views alone are likely to miss this “tilt deformity” (47,48,49,50). Harris (4) was able to expand on this idea with description of mild acetabular dysplasia and pistol grip deformities associated with early osteoarthritis of the hip. Ito et al. (50) coined the term “cam impingement” to describe the consequence of an aspherical junction between the femoral head and neck or an increased radius of the femoral epiphysis as it joints the neck. This was described using an MRI-based anatomical study of the femoral head–neck offset after observing damage to the labrum due to impingement in nondysplastic hips. Patients in the affected group showed a significant reduction in mean femoral anteversion and mean head–neck offset on the anterior portion of the femoral neck, correlating with the site of symptomatic impingement seen on the adjacent rim of the acetabulum during flexion and internal rotation of the hip. Gross abnormalities of the hip are well recognized as precursors to early hip degeneration; however, it was the recognition and recent advances in understanding the more subtle forms of hip abnormalities which are leading to earlier treatment modalities to possibly halt the progression of hip osteoarthritis today.
There are two proposed mechanisms of femoroacetabular impingement: (1) cam impingement caused by a nonspherical femoral head and (2




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree