Agent
Target
Effect
Reference
TGF-β
Mature canine IVD
PG synthesis increased up to 5X; higher in NP than AF
Thompson et al. (1991)
IGF-1
Mature canine IVD
PG synthesis marginally increased in NP
Thompson et al. (1991)
TGF-β
Human annulus cells, 3D culture
Cell proliferation and PG synthesis increased; reduced apoptosis with serum depletion
Gruber et al. (1997)
IGF-1
Young and old bovine NP cells
Cell proliferation and matrix synthesis stimulated; more IGF-1 receptors
Osada et al. (1996)
OP-1
Young rabbit NP and AF cells; alginate beads
Increased PG and collagen production and content
Masuda et al. (2003)
BMP-2
Rat IVD cells monolayer
Increased cell number, GAG, expression for collagen, aggrecan at higher doses
Yoon et al. (2003)
OP-1
Human NP and AF cells, alginate beads
Maintained cell density, increased PG synthesis and accumulation
Imai et al. (2007a)
OP-1
Rabbit IVD cells; alginate beads: IL-1α preexposure
IL-1 decreased PG and collagen; reversed and exceeded with OP-1
Takegami et al. (2002)
OP-1
Rabbit IVD cells; alginate bead: C-ABC preexposure
OP-1 upregulated PG synthesis. Greater effect on C-ABC preexposure than control
Takegami et al. (2005)
BMP-2
Human IVD cells
Increased PG synthesis, expression of aggrecan, collagen types I and II; no bone formation
Kim et al. (2003)
rhBMP-2 and BMP-12
Human IVD cells in monolayer
PG, collagen synthesis increased in NP cells; minimal effect on AF cells
Gilbertson et al. (2008)
GDF-5
Bovine IVD cells; alginate bead
Increased DNA and PG content; at higher dose, PG and collagen synthesis increased
Chujo et al. (2006)
PRP
Porcine IVD cells; alginate bead
Mild increase in cell proliferation; marked increase in PG and collagen synthesis and PG accumulation
Akeda et al. (2006)
TGF-β1 and PRP
Human NP cells
NP cell proliferation and aggregation; increase in mRNA of SOX-9, collagen type II, aggrecan
Chen et al. (2006)
Ad-TIMP-1, Ad-BMP-2
IVD cells from human degenerated IVD
2,000 pg/ml production of TIMP w/100 MOI at day 4. PG synthesis increased with both Ad-TIMP-1 and Ad-BMP-2
Wallach et al. (2003)
Dexamethasone
Human disc herniation tissue explants
Decreased MMP-1 and MMP-3 levels
Genevay et al. (2009)
IL-1ra
Human disc herniation tissue explants
Decreased MMP-3 levels
Genevay et al. (2009)
IL-1ra
Human normal and degenerated disc tissues in situ with IL-1 treatment
IL-1ra reduced cytokine levels (MMP-3, MMP-7, MMP-13) and matrix degradation in all tissue types
Le Maitre et al. (2007b)
IL-1ra/ELP
Human IVD cells (grades 2–3); alginate beads: IL-1ra pre-Tx then IL-1β insult
Reduced ADAMTS-4, MMP-3 transcription
Shamji et al. (2007)
p38 MAPK inhibitor (SB 202190)
Rabbit NP cells pretreated with IL-1
Decreased message for collagen, aggrecan, IGF-1. Increased message for iNOS, COX-2, MMP-3, IL-6
Studer et al. (2008)
TNF inhibitor mAb
Human IVD herniation tissue explants
Decreased MMP-3 levels
Genevay et al. (2009)
PDGF, bFGF, IGF-I
Human NP and AF cells
Increased DNA synthesis via ERK and Akt pathways
Pratsinis et al. (2012)
TGFb3 + Dex, notochordal conditioned media
Degenerated human NP cells
Stimulated NP cell proliferation and decreased ADAMTS-5, MMP-1 expression
Abbott et al. (2012)
Lactoferricin
Bovine NP cells
Increased PG accumulation and expression of SOX-9, aggrecan, TIMP-family genes. Decreased expression of MMPs and ADAMTSs in dose-dependent manner
Kim et al. (2012)
IGF-1, BMP-7, IGF-1 + BMP-7
Bovine NP cells
Synergistically increased anabolic gene expression, PG synthesis, and PG accumulation
Kim et al. (2010)
Table 25.2
The in vivo effects of intradiscal injection treatments
Agent | Species | Site | Model | Effect | Reference |
|---|---|---|---|---|---|
IGF-1 | Rat | Tail | Static compression | Clustering of inner annulus cells after single injection | Walsh et al. (2004) |
GDF-5 | Rat | Tail | Static compression | Clustering of cells, increase in disc height (single injection) | Walsh et al. (2004) |
TFG-β | Rat | Tail | Static compression | Proliferation of cells (multiple injections) | Walsh et al. (2004) |
bFGF | Rat | Tail | Static compression | No response | Walsh et al. (2004) |
OP-1 | Rabbit | Lumbar | None (normal) | Increased disc height and PG content in NP | An et al. (2005) |
OP-1 | Rabbit | Lumbar | C-ABC: co-injection | Increased disc height and PG content in NP | Imai et al. (2003) |
OP-1 | Rabbit | Lumbar | Needle puncture: Tx 4 weeks later | Increased disc height and PG content in NP and AF, improvement of MRI and histology grades | Masuda et al. (2006) |
OP-1 | Rabbit | Lumbar | Needle puncture | Increased disc height and viscoelastic properties | Miyamoto et al. (2006b) |
OP-1 | Rabbit | Lumbar | C-ABC: Tx 4 weeks later | Increased disc height, PG content in NP and AF | Imai et al. (2007b) |
GDF-5 | Rabbit | Lumbar | Needle puncture: Tx 4 weeks later | Increased disc height, improvement of MRI and histology grades | Chujo et al. (2006) |
GDF-5 | Rabbit | Lumbar | Thrombin degraded: Tx 4 weeks later | Increased disc height, improved T1rho and T2 values. Decreased ADAMTS-4 and ADAMTS-5 and COX-2 expression | Bae et al. (2009) |
BMP-2 | Rabbit | Lumbar | Annular stab (5 × 7 mm) | More degeneration, vascularity, and fibroblast | Huang et al. (2007) |
PRP | Rabbit | Lumbar | Nucleotomy, immediate Tx | PRP + GHM group had less degeneration and increased PG; PRP + PBS group showed no differences | Nagae et al. (2007) |
PRP | Rabbit | Lumbar | Nucleotomy, immediate Tx | PRP + GHM had greater disc height, water content, mRNA for PG core protein, and collagen type II; fewer apoptotic cells in NP | Sawamura et al. (2009) |
BMP-17 | Sheep | Lumbar | Annular stab (3 × 6 mm), immediate Tx | BMP-17 maintained disc height, MRI and histology scores, NP cell density; increased PG and collagen synthesis | Wei et al. (2009) |
ADAMTS-5 siRNA | Rabbit | Lumbar | Needle puncture: Tx 4 weeks later | Improved MRI and histology scores | Seki et al. (2009) |
8K-NBD peptide (NF-κB inhibitor) | Mouse | Lumbar | Progeroid Ercc1 p65 KO | Restored total NP GAG and PG synthesis | Nasto et al. (2012) |
allograft MSC or JC in fibrin | Minipig | Lumbar | 1 cm incision and nucleotomy; evaluate at 3, 6, 12 months | JC Tx: high GAG content at 12 months, rich in collagen type II | Acosta et al. (2011) |
Link N protein | Rabbit | Lumbar | Needle puncture: Tx 4 weeks later | Increased aggrecan gene expression and decreased proteinase gene expression | Mwale et al. (2011) |
25.3 Model Systems and Outcome Measures to Evaluate Treatment Efficacy
For evaluation of the efficacy of various biologic treatments, a number of in vitro and in vivo model systems are routinely used. Isolated nucleus pulposus and annulus fibrosus cells from intervertebral discs are seeded in monolayer and treated with specific cytokines (e.g., IL-1, TNF-α) to model degenerative conditions. Cells from discs with varying degrees of degeneration are also used. In these systems, the efficacy of therapeutic agents can be determined at several levels. In cells, a decrease in the gene expression of catabolic enzymes, such as aggrecanases and proteinases, as well as vascular factors and pain-related factors may be assessed using PCR techniques. Anabolic genes of interest may include those molecules comprising the extracellular matrix, such as collagen and aggrecan, and anabolic regulators, such as TGF-β, IGF-1, and growth and differentiation factor-5 (GDF-5). At the protein level, the synthesis of collagen and proteoglycans can be assessed, using radiolabeled 3H and 35S, respectively, by determining their incorporation from the media into the cells. Other factors can be assessed by enzyme-linked immunosorbent assay (ELISA) or multiplex cytokine assay.
Pellets of cells surrounded by three-dimensional extracellular matrices, recovered after alginate culture (Masuda et al. 2000), for example, have also been used. The presence of the extracellular matrix better mimics the microenvironment of disc cells. In addition to treatments using a monolayer system, pellet cultures may be treated with agents to alter or deplete specific matrix components (e.g., using chondroitinase ABC to deplete proteoglycans); this is not feasible using monolayer culture. For outcome measurements, the biochemical content of the matrix can be determined. For example, the dimethylmethylene blue (DMMB) assay can be used to assess the proteoglycan content, while western blot and PCR can be used to assess protein and gene expression levels.
25.4 Effect of Growth Factors on Intervertebral Disc Cells and Tissues
A variety of anabolic growth factors and cytokines alter intervertebral disc homeostasis and stimulate extracellular matrix synthesis (Masuda et al. 2004). OP-1 (Masuda et al. 2003), a member of the BMP family and TGF-β superfamily (Fig. 25.1), upregulates proteoglycan metabolism in intervertebral disc cells. OP-1 strongly stimulated the production and formation of extracellular matrix by rabbit disc cells (Masuda et al. 2003); similar effects have been noted using human intervertebral disc cells (Imai et al. 2007a). OP-1 also replenished proteoglycans and collagens after depletion of the matrix following exposure of intervertebral disc pellets to IL-1 (Takegami et al. 2002) or chondroitinase ABC (C-ABC) (Takegami et al. 2005). The efficacy of OP-1 injection has also been evaluated in a number of in vivo animal models. In adolescent rabbits, an injection of recombinant human OP-1 (rhOP-1), but not the lactose vehicle, reversed the reduction in disc height and improved the magnetic resonance imaging (MRI) grade caused by a needle puncture of the annulus fibrosus (Masuda et al. 2006). In another rabbit study (Miyamoto et al. 2006b), OP-1 restored dynamic viscoelastic biomechanical properties, in needle-punctured intervertebral discs (Miyamoto et al. 2006b). It was also effective in restoring discs that have been chemically degraded with C-ABC, which has been considered as an alternative to chymopapain for chemonucleolysis (Eurell et al. 1990; Fry et al. 1991; Henderson et al. 1991; Kato et al. 1992; Ando et al. 1995; Sugimura et al. 1996; Takahashi et al. 1996b; Yamada et al. 2001), in animal models of disc degeneration such as the rat tail (Norcross et al. 2003; Hoogendoorn et al. 2007, 2008; Boxberger et al. 2008) and goat (Hoogendoorn et al. 2007, 2008). When OP-1 or vehicle was injected into rabbit discs degraded with C-ABC for 4 weeks, the disc height was initially decreased (∼34 %), then recovered, and gradually approached the level of the control (Imai et al. 2007b).
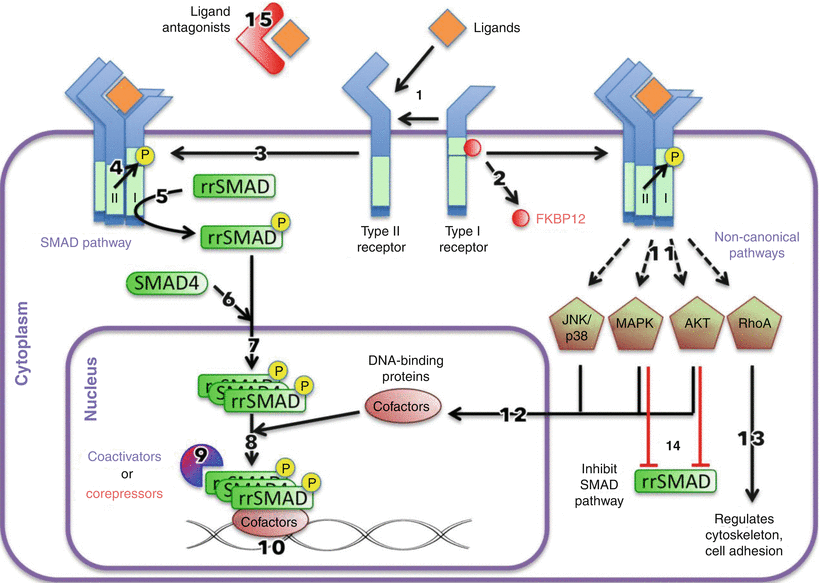

Fig. 25.1
TGF-β signaling pathways. Ligand binds with types I and II receptors (1), which releases FKBP12 (2) from the GS domain of the type I receptor and forms a ligand-receptor complex (3). The type I receptor is phosphorylated by type II receptor (4). The activated type I receptors phosphorylate receptor-regulated SMADs (rrSMAD) (5), which associate with SMAD4 (6) and move into the nucleus (7). The SMAD complex associates with DNA-binding cofactors (8) along with coactivators or corepressors (9) to activate or repress transcription of genes (10). The activated ligand-receptor complex can begin other noncanonical pathways (11), which can affect cofactors (12), regulate cytoskeletal organization and cell adhesion (13), or inhibit the SMAD pathway (14). In addition, ligand antagonists can sequester ligands extracellularly (15)
A number of autologous agents have been shown to be clinically useful. For example, platelet-rich plasma (PRP) contains high levels of multiple growth factors and as such it has been used in disc repair in animal studies and in a clinical study ongoing in Japan. Moreover, PRP can be easily generated in the operating room by centrifugal separation of autologous blood using a point-of-care device. In vitro, PRP-stimulated porcine disc cell proliferation and matrix synthesis (Akeda et al. 2006) and using isolated human disc cells provoked the formation of a nucleus pulposus-like tissue (Chen et al. 2006). The efficacy of injecting allograft PRP with or without a gelatin hydrogel (which provides slow release and mechanical support) was explored using a rabbit model of nucleotomy (Nagae et al. 2007). The PRP hydrogel was found to markedly suppress further degeneration, compared to PRP alone and a saline control group. A follow-up study suggested that the hydrogel microspheres without PRP did not have therapeutic value. In contrast, animals treated with PRP with microspheres benefited from increased disc height, elevated water content, increased expression of proteoglycan core protein and collagen II, and fewer apoptotic cells in the nucleus pulposus (Sawamura et al. 2009). In less severe models without nucleotomy, PRP (after activation with autologous serum and calcium) injection alone has been effective for disc repair (Obata et al. 2012).
In addition to PRP and OP-1, many other anabolic growth factors and cytokines have been investigated. Early studies indicated that TGF-β promoted disc cell proliferation (Gruber et al. 1997) and stimulated proteoglycan synthesis (Thompson et al. 1991; Gruber et al. 1997). IGF-1 (Osada et al. 1996; Pratsinis and Kletsas 2007) and platelet-derived growth factor (PDGF) (Pratsinis and Kletsas 2007) also showed a similar cell-proliferative effect. In addition, PDGF exerted a protective, antiapoptotic effect on annulus fibrosus cells induced by serum depletion (Gruber et al. 2000).
Box 25.1 Platelet-Rich Plasma
Platelet-rich plasma (PRP) injection is increasingly used as an off-label procedure before resorting to orthopedic surgery. PRP contains a mixture of growth factors (Weibrich et al. 2002; Okuda et al. 2003; Dugrillon et al. 2002; Mazzocca et al. 2012) such as transforming growth factor (TGF)-β1 and TGF-β2, vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and insulin-like growth factor (IGF), which are naturally released from platelets after being activated by calcium or thrombin (Tozum and Demiralp 2003; Arpornmaeklong et al. 2004). Among these growth factors, TGF-β1 exists in the highest concentration (Weibrich et al. 2002) and may be the core ingredient and the indicator for applying PRP. These growth factors appear to play an important role in wound healing and are assumed to facilitate hard and soft tissue regeneration.
The efficacy of PRP injection for disc regeneration is still being investigated; however, promising results such as upregulation of proteoglycan anabolism and restoration of disc height have been seen in a number of in vitro studies on disc cell (Akeda et al. 2006; Chen et al. 2006), on explants (Chen et al. 2009), as well as in animal models (Nagae et al. 2007; Chen et al. 2009; Obata et al. 2012). PRP is often used in conjunction with biomaterials such as gelatin (Nagae et al. 2007) and mesenchymal stem cells (Chen et al. 2006, 2009). PRP has been used without activation (Nagae et al. 2007); however, most commercial systems (Castillo et al. 2011) use PRP releasate.
25.5 Effect of Other Modalities on Intervertebral Disc Cells and Tissues
Agents other than anabolic growth factors are being considered for repair of the degenerate intervertebral disc. In a recent study, ADAMTS-5 expression was silenced using siRNA (Seki et al. 2009). In vitro studies, using rabbit nucleus pulposus cells, were performed to determine the extent of reduction of ADAMTS-5 expression. Then, using an annular needle puncture rabbit model, MRI and histological analysis were used to assess the efficacy of ADAMTS-5 siRNA to prevent tissue degradation. After 8 weeks, the control group exhibited a complete loss of nucleus pulposus tissue, while the ADAMTS-silenced animals maintained disc structure (Seki et al. 2009).
IL-1 receptor antagonist (IL-1ra) is another inhibitory agent that has been studied. When applied in vitro to degenerated (Le Maitre et al. 2007b) and herniated (Genevay et al. 2009) human disc tissues, IL-1ra reduced the expression of MMP-3 (Le Maitre et al. 2007b; Genevay et al. 2009). In addition, following pretreatment of degenerated human nucleus pulposus cells with IL-1ra and subsequent treatment with IL-1, there was reduced expression of ADAMTS-4 and MMP-3 (Shamji et al. 2007).
Yet another target for inhibition is TNF-α. In human disc cells, the use of soluble TNF receptors along with IL-1ra significantly upregulated proteoglycan synthesis (Kakutani et al. 2008), while co-treatment with TNF-α suppressed nitric oxide and IL-6 production (Sinclair et al. 2011). Although dominant-negative TNF (DN-TNF) does not activate TNF receptors, it effectively competes with soluble TNF. When applied to human intervertebral disc cells challenged with IL-1β (Pichika et al. 2011), DN-TNF effectively reduced the concentration of TNF-α (Fig. 25.2a, b), levels of MMP-3 in the media (Fig. 25.2c, d), the expression of intracellular caspase 3 (Fig. 25.2e, f), and the production of PGE2. The use of a monoclonal antibody against TNF-α suppressed the expression and concentration of MMP-3 in explants of herniated discs (Genevay et al. 2009). Other TNF-inhibiting agents are beginning to be used clinically for analgesic purposes in patients with sciatica (Karppinen et al. 2003; Genevay et al. 2004; Okoro et al. 2010) and discogenic pain (Tobinick and Britschgi-Davoodifar 2003). Anti-cytokine therapeutics include the p38 mitogen-activated protein kinase (MAPK) inhibitor, which suppressed MMP-3 and IL-1 expression (Studer et al. 2008) as well as a NF-κB decoy, which reduced pain in a rat lumbar disc herniation model (Suzuki et al. 2009).
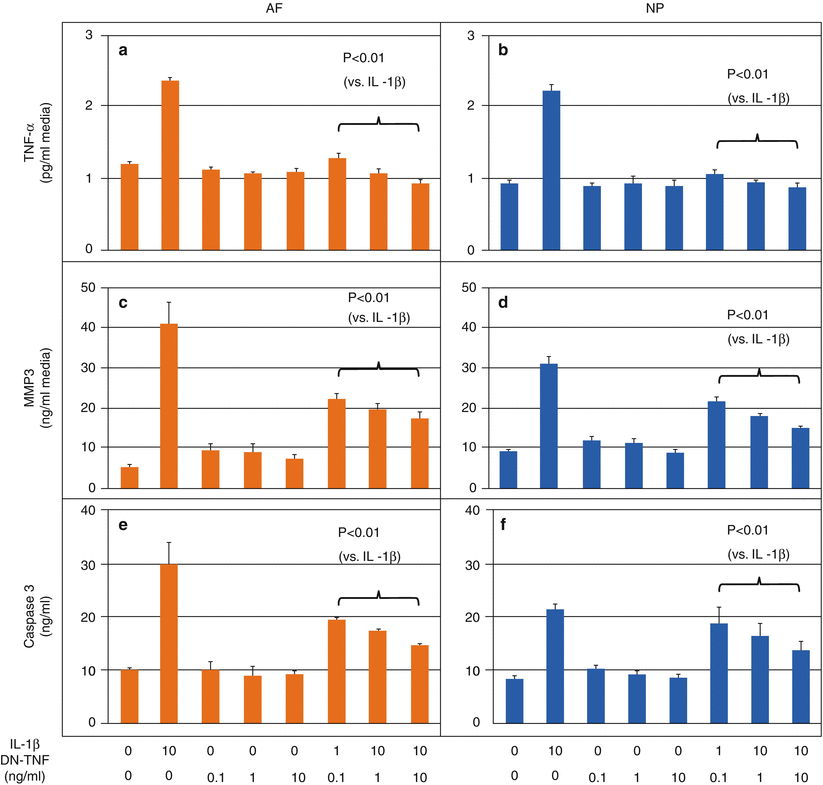

Fig. 25.2
Dominant-negative tumor necrosis factor (DN-TNF) effectively antagonizes interleukin-1β (IL-1β)-induced catabolic changes in human intervertebral disc cells. Human nucleus pulposus (NP) and annulus fibrosis (AF) cells were isolated from cadaveric intervertebral disc (IVD) tissues (average of ∼60 years old) and cultured in alginate beads for 7 days. Cells were serum starved for 1 day and treated for 2 days in media containing a combination of IL-1β and DN-TNF. Levels of TNF-α, matrix metalloproteinase-3 (MMP-3), and caspase 3 released into the media were measured. DN-TNF treatment suppressed release of (a, b) TNF-α, (c, d) MMP-3, and (e, f) caspase 3 by both AF and NP cells, suggesting DN-TNF may be effective in suppressing catabolic cytokines, matrix-degrading enzymes, and cell apoptosis (Modified from Pichika et al. (2011))
Although the exact mechanism is unclear, the use of gelatinous biomaterials, alone or in combination with growth factors, has shown some efficacy. These materials may work by providing immediate load support (Joshi et al. 2005) as well as a hydrated environment in which host or embedded cells can proliferate (Collin et al. 2011). Fibrin glue has been used to repair intervertebral discs in a porcine nucleotomy model: it suppressed IL-6 and TNF expression and restored mechanical properties and the glycosaminoglycan (GAG) content (Buser et al. 2009). Hyaluronic acid-cross-linked hydrogels have been used in rabbits with less severe annular needle punctures and were found to improve disc height (Fig. 25.3a) and T2 MR properties (Bae et al. 2011) (Fig. 25.3a–d), as well as safranin O staining (Nakashima et al. 2009). Mixing the hydrogel with TGF-β prior to injection further increased disc height (Fig. 25.3a) (Bae et al. 2011). While little is known about degradation of these biomaterials in vivo and their long-term biologic effects, the use of a suitable carrier material may synergize growth factor activity.
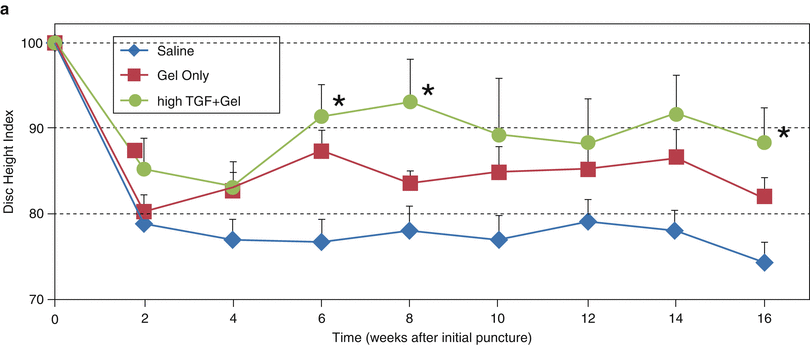
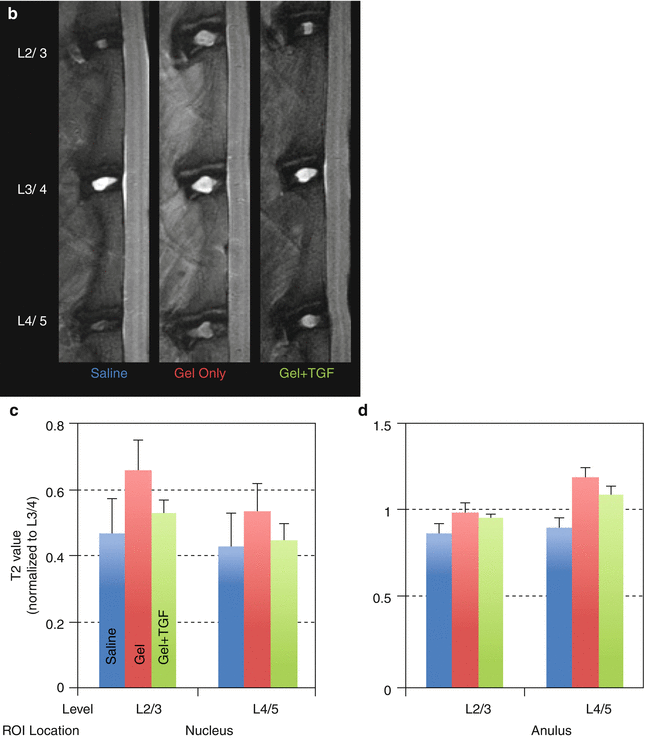


Fig. 25.3
Treatment of intervertebral disc degeneration using injectable hydrogel. Adult (∼9 months old) rabbits received annular puncture. After 4 weeks, animals were divided into three groups and received an injection of either saline, hyaluronic acid-based hydrogel, or transforming growth factor β3 (TGFβ3) mixed with the hydrogel. Lateral plane radiographs were taken biweekly. At week 16, animals were sacrificed and MRI was performed to determine T2 relaxation properties of the discs. (a) Disc height index was higher for hydrogel samples than saline samples, consistent with (b) MRI showing larger nucleus pulposus (NP) morphology for the hydrogel samples. (c) T2 property of the NP also showed a trend of being higher for the hydrogel group, while (d) that of the AF did not show much variation. The addition of TGFβ3 increased disc height even more (a) but had no effect on the T2 property. ROI region of interest (Reproduced from Bae et al. (2011), abstract)
While of limited use for development of injectable therapeutic agents, a mechanism for stimulating disc cells anabolism would be of great value for countering the in vivo degradation of intervertebral disc tissues. One factor that would be expected to impact anabolic events is local oxygen tension; in the disc this is low, between 2 and 5 % in vivo (Urban 2002; Bartels et al. 1998). Low oxygen tension would be expected to decrease mitochondrial function and oxidative activity (Bibby et al. 2005). It has been observed that it enhanced the anabolic effects of OP-1 (Miyamoto et al. 2006a), BMP-7 (Tonomura et al. 2007), and TGF-β3 (Abe et al. 2008) by promoting extracellular matrix synthesis by bovine nucleus pulposus cells. In addition, in MSCs (Stoyanov et al. 2011) and notochordal cells (Erwin et al. 2009), low oxygen tension has been shown to facilitate differentiation and extracellular matrix production. This topic is discussed further in Chap. 6.
Low oxygen tension results in lactate accumulation and a concomitant decrease in media pH (Pichika et al. 2012). Dynamic mechanical stimulation in a physiologic range can also stimulate anabolic activities. Thus, applying cycling tensile strain to discs resulted in F-actin reorganization and an increase in collagen I expression in outer annulus fibrosus cells and an increase of collagen II expression in the nucleus pulposus (Li et al. 2011). Rat caudal discs compressed in vivo at 1.0 MPa stress and 0.01 Hz frequency exhibited increased expression of anabolic genes (Maclean et al. 2004). For tissue-engineering applications, these techniques may be combined with growth factors and scaffold or gel biomaterials to help optimize the function of disc tissues (this topic is discussed in detail in Chap. 26).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








