Disorders of the Cervical Spine
Jason S. Lipetz
David I. Lipetz
The intricately designed and highly mobile cervical spine is routinely subject to unique mechanical stresses that predispose the spinal elements to degenerative change. Three common symptom complexes are associated with the degenerative cascade of the cervical spine. These include cervical axial pain, radiculopathy, and myelopathy (1, 2). These syndromes may occur in isolation or in combination. Axial pain, arising from degenerative discogenic or zygapophyseal joint pain generators, is more commonly described in the middle decades of life (3). Spinal nerve root pathology and radicular syndromes may arise from an acutely offending disc injury or more gradually evolving and degenerative neural foraminal compromise (4, 5). Myelopathic symptoms occur when the spinal cord is affected by central canal stenosis (6, 7, 8). This chapter will review these common degenerative cervical syndromes, the cervical spine in rheumatoid arthritis (RA), and rehabilitative approaches.
ANATOMY AND BIOMECHANICS
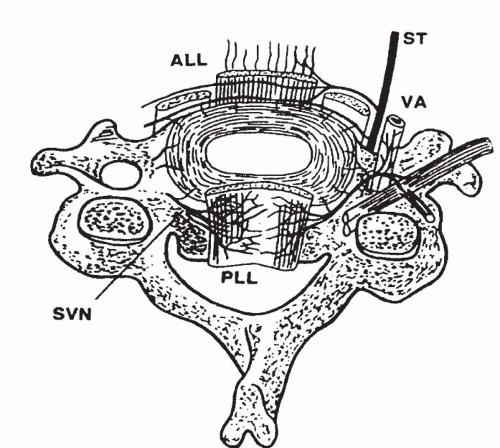
The cervical spine is composed of seven vertebrae and five intervertebral discs. The C1 and C2 segments are anatomically unique. The C2 through C7 bodies articulate anteriorly through the intervertebral discs and uncovertebral joints, or joints of Luschka, and posteriorly through the zygapophyseal joints (Fig. 32-1). The occipito-atlanto-axial or Oc-C1-C2 complex is a specialized upper cervical segment that allows for a significant range of motion between the head and upper torso (9). The articulations of this segment are composed of synovial joints with no intervertebral discs. The basic structure of this segment is a biconcave ring of C1 interposed between the convex condyles of the occiput (Oc) above and the C2 lateral masses below. The odontoid, or dens, projects upward from the C2 vertebrae, providing a post to which the C1 ring and Oc are directly anchored (9). The Oc is attached to the odontoid predominantly by the alar ligaments and also by the less significant apical and upper arms of the cruciform ligament (10). The C1 ring is bound to the odontoid by the sturdy transverse arm of the cruciform ligament as well as the accessory C1-2 ligaments and the C1-2 joint capsules (11) (Fig. 32-2).
In the lower cervical spine, the intervertebral discs contribute to approximately one fourth of the height of the cervical column (12, 13). The dimensions of the cervical disc, which is thicker anteriorly than posteriorly, contribute to the cervical spine’s lordotic curvature (14). The cervical disc allows for a greater degree of motion than do the discs in the lumbar region, as the disc-to-vertebral body height ratio in the cervical region is 2:5, compared with a 1:3 ratio in the lumbar spine. The anterior longitudinal ligament runs along the anterior vertebral body and discs, providing structural support in limiting cervical extension. The posterior longitudinal ligament supports the disc and body posteriorly, stretching in flexion and relaxing with extension. The superficial ligamentum nuchae is a dense midline band that extends from the Oc to the spinous process of C7. Proceeding ventrally in the midsagittal plane, the supraspinous ligament, interspinous ligament, and ligamentum flavum are encountered in order, each contributing to stability in flexion (9).
The external component of the intervertebral disc is comprised of the annulus fibrosus, which consists of multiple lamellae of type I and II collagen. The annulus encapsulates the internal nucleus pulposus. The fiber direction of the annular lamellae alternates, with each consecutive layer oriented opposite to the adjacent fibers (14). This structural arrangement uniquely allows the annulus to accommodate angular motion while providing stability against torsion and shear. The intervertebral discs are separated from the vertebral bodies by the end plates (13, 15). The end plates are composed of hyaline and fibrocartilage and form a permeable surface through which nutrients may pass between the cancellous bone of the vertebral body and the intervertebral discs. The end plates are vascularized during fetal life, but their vessels involute during the first 10 to 15 years of development (16). Thereafter, the essentially avascular intervertebral disc receives its nourishment through the end plates and vessels circumscribing the outer annular fibers (12, 14). The nucleus pulposus is a semifluid gel, composed predominantly of water, which represents 40% to 60% of the intervertebral disc and allows for deformation and accommodation of movement and compressive loading. With aging, the nucleus is slowly replaced by fibrocartilage, and by the end of the first decade of life, the distinction between the nucleus and surrounding annulus begins to lessen (12). The innervation of the outer one third to half of the annulus fibrous has been demonstrated (17, 18). The anterior disc is innervated by branches of the vertebral nerves and sympathetic
trunks. The posterior disc, posterior longitudinal ligament, anterior dura, and dural root sleeves are innervated by a posterior plexus derived from the sinuvertebral nerves (19) (Fig. 32-3).
trunks. The posterior disc, posterior longitudinal ligament, anterior dura, and dural root sleeves are innervated by a posterior plexus derived from the sinuvertebral nerves (19) (Fig. 32-3).
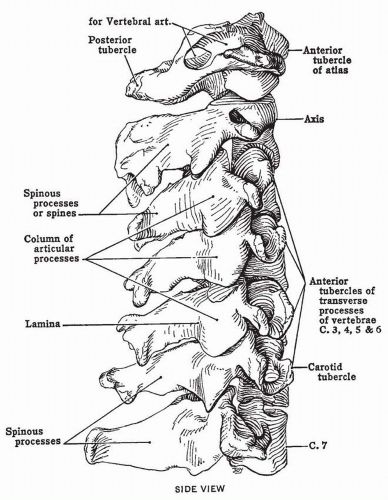 FIGURE 32-1. Articulated cervical vertebrae: lateral view. (From Anderson JE. Grant’s Atlas of Anatomy. 8th ed. Baltimore, MD: Williams & Wilkins; 1983, with permission.) |
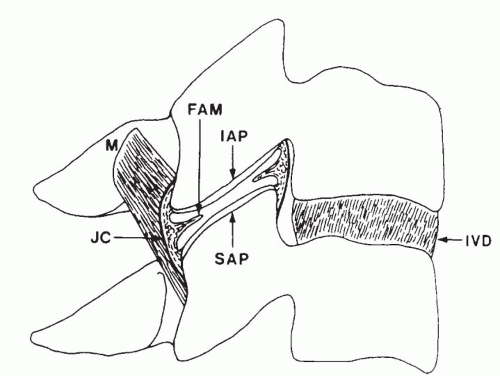
The uncovertebral joints or joints of Luschka are anterior articulations found between the third through seventh vertebrae that share in load bearing and are often affected by degenerative change (12). The zygapophyseal joints are planar synovial joints, which are created by the inferior and superior articular processes of adjacent vertebrae. Each joint is composed of circular or ovoid facets that are covered by articular cartilage and enclosed by a fibrous joint capsule (20). The zygapophyseal joint may include fibroadipose menisci located at the ventral and dorsal joint poles. These menisci are thought to be drawn from the joint cavity during joint gliding to cover those articular surfaces that become exposed during such motions (21) (Fig. 32-4). The cervical zygapophyseal joints are innervated by articular branches originating from the medial branches of the cervical dorsal rami. Each cervical zygapophyseal joint receives a dual innervation from the medial branches arising from the dorsal rami above and below. The medial branches of the C3 dorsal ramus are unique. A deep C3 medial branch contributes to the C3-4 joint innervations, whereas the larger superficial medial branch of C3, also known as the third occipital nerve, innervates the C2-3 joint (20). Beyond the C2-3 joint, the third occipital nerve innervates the semispinalis capitis and supplies cutaneous sensation to the suboccipital region. This is the only dorsal ramus below C2 that has a cutaneous distribution (22).
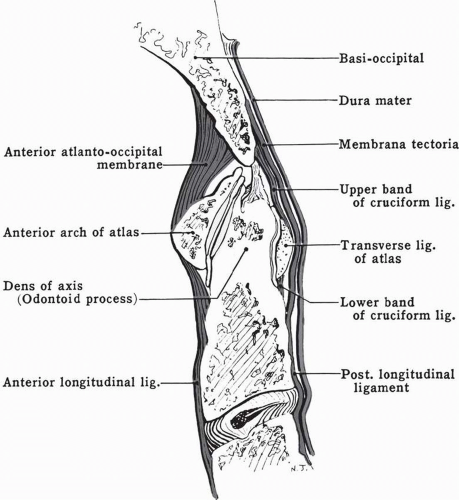 FIGURE 32-2. Median section of Oc C1-2 complex. (From Anderson JE. Grant’s Atlas of Anatomy. 8th ed. Baltimore, MD: Williams & Wilkins; 1983, with permission.) |
From C2-3 through C5-6, the zygapophyseal joints are angled at approximately 45 degrees, with the longitudinal axis of the cervical spine, with a typically steeper angle at C6-7 (23). The superior articular processes are progressively taller at the more caudal segments, with the upper ends of the joints extending further above the level of the segmental intervertebral disc. The orientation of the zygapophyseal joints allows them to resist both forward and downward displacement of the vertebral body. As the zygapophyseal joint of the upper cervical segments are relatively more horizontally oriented, they contribute more to bearing axial loads than do the more caudal segments. The orientation of the zygapophyseal joints dictates the nature and magnitudes of movement in the cervical spine (20).
The primary motion of the Oc-C1-C2 complex is flexion and extension at both Oc-C1 and C1-2, axial rotation only at C1-2, and minimal lateral bending at Oc-C1 (9). The Oc-C1 articulation allows for approximately 10 degrees of flexion and 25 degrees of extension. Rotation of 45 degrees in either rotation can be observed at the C1-2 joint (24, 25). A useful approximation of the range of motion allowed by each segment between C2-3 and C7-T1 is 10 degrees of total range from flexion to extension, as well as 10 degrees of lateral bending and axial
rotation to each side. Flexion and extension and axial rotation tend to be the greatest in the midcervical region, decreasing both above and below. Lateral bending is typically greatest at C2-3 and diminishes caudally (26). Instability of the cervical region has been defined as a horizontal displacement of the vertebral body during flexion and extension of greater than 3.5 mm or angular change of greater than 11 degrees (27).
rotation to each side. Flexion and extension and axial rotation tend to be the greatest in the midcervical region, decreasing both above and below. Lateral bending is typically greatest at C2-3 and diminishes caudally (26). Instability of the cervical region has been defined as a horizontal displacement of the vertebral body during flexion and extension of greater than 3.5 mm or angular change of greater than 11 degrees (27).
The neural elements of the cervical spine include the spinal cord, dorsal and ventral roots, spinal nerves, and dorsal and ventral rami. The dural and arachnoid mater sleeve contain the ventral and dorsal roots and ultimately blend with the spinal nerve epineurium. The spinal nerves are formed by the joining of the ventral and dorsal roots. Each spinal nerve exits the spinal canal through the intervertebral foramen, which is bordered by the uncovertebral joint anteromedially, the zygapophyseal joint posteriorly, and superiorly and inferiorly by the pedicles of the respective vertebral bodies. The most cephalad and first true neural foramen is located at the C2-3 level. This foramen has the largest area, with a progressive reduction in foraminal size observed more caudally (13). The spinal nerve is a mixed nerve that resides within the neural foramen and is accompanied by the radicular arteries and veins (28). The C3 through C7 nerves exit above their respective pedicles, whereas the C8 spinal nerve exits beneath the pedicle of C7. The C1 nerve rests on the posterior arch of C1, where it divides into dorsal and ventral rami, and the C2 nerve exits the thecal sac and descends obliquely across the dorsal aspect of the atlantoaxial joint (29). Upon exiting the intervertebral foramen, the spinal nerves divide into dorsal and ventral rami. The C5-T1 ventral rami contribute to the brachial plexus. The C1-4 ventral rami comprise the cervical plexus, which innervates the cervical musculature and cutaneous structures of the ear, face, and neck. The C1 and C2 ventral rami innervate the atlanto-occipital and atlantoaxial joints, respectively (19).
AXIAL PAIN AND SYMPTOM REFERRAL
Epidemiology
Cervical spondylosis describes the degenerative change that affects the five articulations of the cervical segment, including the intervertebral discs, the bilateral zygapophyseal joints, and the uncovertebral joints of Luschka (30). The degenerative cascade is thought to begin with disc desiccation and loss of disc height, which is followed by an approximation of the uncovertebral joints and a disruption of the normal zygapophyseal joint biomechanics. Uncovertebral and zygapophyseal joint hypertrophy, osteophyte formation, anular disruption, and ligamentum flavum hypertrophy can comprise the ensuing phases of degenerative change (31, 32, 33). Radiographic evidence of degenerative change is appreciated in 10% of individuals by the age of 25, 35% by age 40, and in up to 95% by the age of 65 (34, 35, 36). Plain film imaging in asymptomatic subjects reveals degenerative change affecting the cervical spine in 70% of women and 95% of men between the ages of 60 and 65 (37). It is essential to remember that although there is typical radiographic evidence of degenerative disease, including loss of disc space height and age-dependent osteophytes, in individuals presenting with cervical pain, not
all individuals with degenerative findings are symptomatic (3, 37). The incidence of neck pain similarly increases with age, progressing in a fairly linear fashion from age 20 through 60 (3, 38). Approximately 95% of individuals will experience cervical pain by the age of 65 (35). Severe episodes of neck discomfort will affect 10% of the population at some point in their lives. The annual incidence of significant cervical pain has been estimated at 12.3% (36).
all individuals with degenerative findings are symptomatic (3, 37). The incidence of neck pain similarly increases with age, progressing in a fairly linear fashion from age 20 through 60 (3, 38). Approximately 95% of individuals will experience cervical pain by the age of 65 (35). Severe episodes of neck discomfort will affect 10% of the population at some point in their lives. The annual incidence of significant cervical pain has been estimated at 12.3% (36).
Referred pain is pain perceived in a region innervated by nerves other than those innervating the true source of the pain. Pain referral patterns arise as the brain is unable to decipher the true pain origin secondary to convergence at the level of the spinal cord and thalamus. Referred pain is typically experienced as deep, diffuse, and poorly localized pain (19). Any of the innervated structures of the cervical spine can contribute to local pain as well as symptom referral. Although injury to the cervical musculature and ligamentous structures can result in local and regional pain (39, 40), the intervertebral disc and zygapophyseal joints have been more extensively investigated as pain generators.
Intervertebral Disc
Degenerative disc disease is most commonly observed at C5-6, with C6-7 involvement the next most prevalent. These intervertebral discs might be more commonly affected because of increased segmental motion at these spinal segments (41, 42). An injured disc, resulting from degenerative change or more acute trauma, can produce local and referred symptoms (17). The outer third of the annulus fibrosus houses nerve endings that can be stimulated during injury. It has been suggested that degenerative or traumatic alteration of the internal architecture of the annulus can result in pain production through stimulation of local mechanoreceptors and nociceptors (17, 41). The ability of the annulus to mediate pain has been demonstrated during surgery through mechanical and electrical stimulation of cervical intervertebral discs (42). In addition to this mechanical component of disc injury, discogenic pain might also be biochemical in origin. An annular defect could allow for the migration of nuclear material, which might then stimulate the outer annulus, dura mater, posterior longitudinal ligament, dorsal root ganglion, or spinal nerve (43). Increased levels of inflammatory mediators have been identified in degenerative and herniated discs when compared with asymptomatic controls (44).
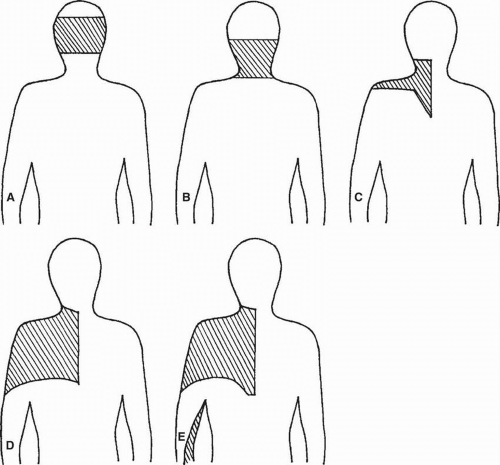
Using cervical discography, characteristic discogenic pain patterns have been described (42, 45, 46). The correlation between cervical disc magnetic resonance imaging (MRI) abnormalities and pain-generating potential observed through discography has been shown to be poor (46). In a study of 807 disc injections, 404 concordant pain responses were used to describe pain referral patterns. Pain referral to the scapular region was observed to arise from disc stimulation at the C3-4 through C7-T1 levels. The C5-6 and more caudal segments were noted to refer symptoms to the upper limb, and C6-7 stimulation was unique in that pain was referred to the anterior chest wall (45) (Fig. 32-5).
Zygapophyseal Joints
The zygapophyseal joints can also become active pain generators in the setting of degenerative change or following trauma.
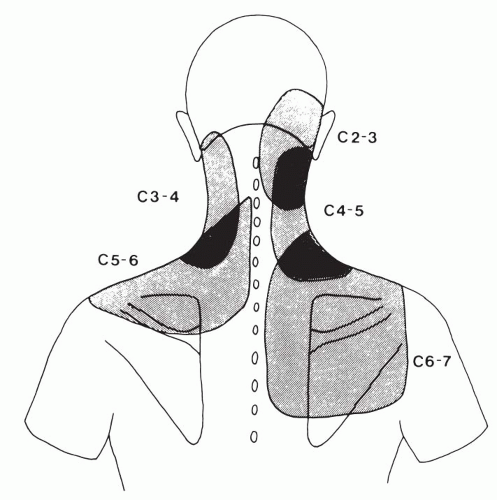
These posterior elements are particularly vulnerable to injury during a whiplash event. Although often not observed radiographically, zygapophyseal joint fractures, intra-articular hemorrhage, and capsular tears have been observed in pathologic studies (47, 48, 49, 50, 51, 52, 53). The prevalence of whiplash-induced zygapophyseal joint pain has been described (54, 55, 56). Using diagnostic blocks, chronic cervical zygapophyseal joint pain has an estimated frequency of 54% to 60% (55, 56). In this chronic neck pain population, the C2-3 joint has been observed to be most frequently symptomatic, followed by C5-6 (55, 56). Of those patients with chronic neck pain, 58% to 88% describe significant headaches (54, 55, 56). The prevalence of C2-3 jointmediated headaches has been estimated at 50% to 53% in those individuals with a chief symptom of headache following whiplash (54, 56). The Oc-C1 joints (54, 57, 58, 59) and C1-2 joints (57, 58) have similarly been described as active nociceptors in cervicogenic headaches. Studies in a limited number of asymptomatic and symptomatic volunteers have suggested particular resultant pain patterns from intraarticular zygapophyseal joint stimulation and anesthetization (60, 61, 62). Pain arising from C2-3 stimulation was described as referred to the upper neck and occipital region, whereas C3-4 and C4-5 joint pain was observed to affect the mid- and lower cervical region, extending toward the superior scapular border. Pain from C5-6 stimulation was observed to extend toward the shoulder, whereas C6-7 pain radiated over the more caudal scapular region (Fig. 32-6). These degenerative or posttraumatic pain referral patterns arising from primary axial zygapophyseal joint or discogenic pain generators can be difficult to distinguish clinically from one another and from radicular pain presenting without a more distal component or neurologic correlate.
These posterior elements are particularly vulnerable to injury during a whiplash event. Although often not observed radiographically, zygapophyseal joint fractures, intra-articular hemorrhage, and capsular tears have been observed in pathologic studies (47, 48, 49, 50, 51, 52, 53). The prevalence of whiplash-induced zygapophyseal joint pain has been described (54, 55, 56). Using diagnostic blocks, chronic cervical zygapophyseal joint pain has an estimated frequency of 54% to 60% (55, 56). In this chronic neck pain population, the C2-3 joint has been observed to be most frequently symptomatic, followed by C5-6 (55, 56). Of those patients with chronic neck pain, 58% to 88% describe significant headaches (54, 55, 56). The prevalence of C2-3 jointmediated headaches has been estimated at 50% to 53% in those individuals with a chief symptom of headache following whiplash (54, 56). The Oc-C1 joints (54, 57, 58, 59) and C1-2 joints (57, 58) have similarly been described as active nociceptors in cervicogenic headaches. Studies in a limited number of asymptomatic and symptomatic volunteers have suggested particular resultant pain patterns from intraarticular zygapophyseal joint stimulation and anesthetization (60, 61, 62). Pain arising from C2-3 stimulation was described as referred to the upper neck and occipital region, whereas C3-4 and C4-5 joint pain was observed to affect the mid- and lower cervical region, extending toward the superior scapular border. Pain from C5-6 stimulation was observed to extend toward the shoulder, whereas C6-7 pain radiated over the more caudal scapular region (Fig. 32-6). These degenerative or posttraumatic pain referral patterns arising from primary axial zygapophyseal joint or discogenic pain generators can be difficult to distinguish clinically from one another and from radicular pain presenting without a more distal component or neurologic correlate.
A randomized controlled study investigating the therapeutic benefit of intra-articular zygapophyseal joint injections using corticosteroid has suggested that such treatment is ineffective (63). A retrospective uncontrolled study of intra-articular C2-3 joint injection in the treatment of post-traumatic headaches has suggested a significant therapeutic response. A randomized placebo controlled trial of radiofrequency neurotomy in patients with unremitting cervical zygapophyseal joint pain has demonstrated a significant treatment effect (64). Pain is expected to return following neurotomy, as distal axons innervating the zygapophyseal joint are likely to regenerate.
RADICULOPATHY
Epidemiology
Cervical radiculopathy can arise from pathologic compressive processes affecting the nerve root, including acute disc herniation, degenerative foraminal stenosis, trauma, or tumor (65). In the 1920s, cervical nerve root compression was described as a cause of anginoid pain (66). Cervical disc herniation resulting in cord compression was initially recognized as a syndrome of spinal cord tumors or chondromas. This syndrome of cord compression was subsequently defined by Mixter and Ayer in 1935 (67) as arising from a ruptured intervertebral disc. In 1936, cervical spondylosis and neural irritation was described as a cause of shoulder girdle and arm pain (68). In the early 1940s, cervical nerve root irritation arising from disc injury was defined (69).
The annual incidence of cervical radicular pain is 5.5/100,000 (70). These injuries represent 5.3% of all nerve root injuries caused by disc pathology. The nerve roots most commonly involved by cervical radiculopathy are C7 and C6, with most studies suggesting C7 syndromes as the most common, followed by radiculopathy of C5 and C8 (65, 71, 72, 73, 74). Patients younger than 55 years are more likely to present with radiculopathy arising from acute disc herniations, whereas those older than 55 years are more likely to demonstrate symptoms arising from degenerative foraminal or central canal stenosis (30).
Pathophysiology
The cervical nerve root can be compressed in the neural foramen by the intervertebral disc, degenerative change affecting the zygapophyseal or uncovertebral joint, or in a combined fashion. Cervical radiculopathy arising from a herniated disc is more common than radiculopathy arising from the more slowly evolving degenerative foraminal stenosis typically observed in older individuals (75, 76, 77, 78) (Fig. 32-7). Disc herniations have been classified as “soft” and “hard.” “Soft” refers to more acute disc injuries and displacement of nucleus pulposus
material, whereas “hard” disc material describes a calcified and spondylotic ridge (72, 79, 80). With disc degeneration, tears in the annulus can allow for the displacement of the nucleus. Anterior disc herniations are less common as the anterior longitudinal ligament is wider and stronger than the posterior longitudinal ligament (30).
material, whereas “hard” disc material describes a calcified and spondylotic ridge (72, 79, 80). With disc degeneration, tears in the annulus can allow for the displacement of the nucleus. Anterior disc herniations are less common as the anterior longitudinal ligament is wider and stronger than the posterior longitudinal ligament (30).
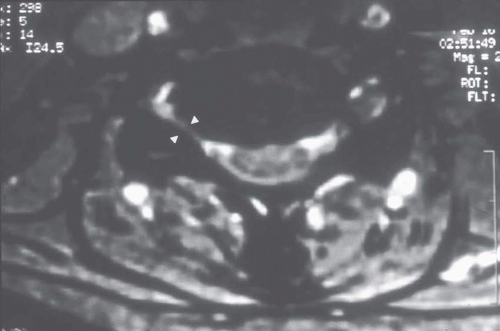
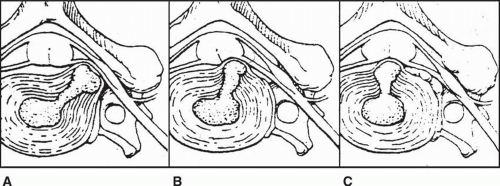
Disc herniations have been characterized based on location (81, 82). The three locations described are intraforaminal, posterolateral, and central. Intraforaminal herniations are the most common and may result in an acute radiculopathy affecting the nerve root exiting through the respective foramen. Therefore, an intraforaminal herniation of disc material at the C4-5 level might result in a C5 radiculopathy. The posterolateral disc herniation occurs because the rhomboidal shape of the posterior longitudinal ligament tends to direct disc material to one side or the other. These herniations are located between the lateral edge of the posterior longitudinal ligament and the posterior aspect of the uncinate process. The central disc herniation passes through the substance of the posterior longitudinal ligament. Such lesions are more likely to result in central canal compromise and spinal cord compression. Central herniations are believed to be more likely to occur in the later stages of cervical degeneration when uncovertebral joint hypertrophy serves as a barrier to the more lateral migration of disc material (83) (Fig. 32-8).
The resultant injury to the nerve roots likely arises from combined mechanical and biochemical pathophysiologic processes. A study in which cervical disc specimens removed surgically from patients with radiculopathy were compared with discs from a traumatic control group found significantly increased levels of matrix metalloproteinase, nitric oxide, prostaglandins, and interleukins in the radiculopathy group (44). These findings, in conjunction with a plethora of literature describing the combined mechanochemical nature of lumbar radicular syndromes, suggest that these biochemical markers are likely involved in the injury process of cervical radiculopathy (84, 85, 86, 87, 88).
Radiologic Findings
Plain films can be used as an initial screening tool but are not necessary in every individual presenting with cervical or radicular pain. In addition to anteroposterior and lateral images, flexion and extension views can prove of value in patients with recent trauma, suspected instability, or in cases of ankylosing spondylitis or RA (65).
MRI is considered the imaging modality of choice in cervical radiculopathy (89). Earlier comparative studies demonstrated a close correlation between findings on cervical MRI, computed tomography (CT) scan, and myelography (90, 91, 92). More recent studies have suggested a higher correlation between radiographic and surgical pathology when MRI was used than with either myelography (93) or CT (94). A high incidence of abnormalities has been observed on cervical MRI imaging of asymptomatic individuals. Boden et al. (95) observed significant abnormalities in 19% of 63 asymptomatic patients.
In those younger than age 40, 14% had a herniated disc or neural foraminal stenosis, and similar abnormalities were observed in 25% of individuals older than age 40 (83). An investigation of cervical myelography has identified nerve root filling defects in 21% of asymptomatic subjects (96).
In those younger than age 40, 14% had a herniated disc or neural foraminal stenosis, and similar abnormalities were observed in 25% of individuals older than age 40 (83). An investigation of cervical myelography has identified nerve root filling defects in 21% of asymptomatic subjects (96).
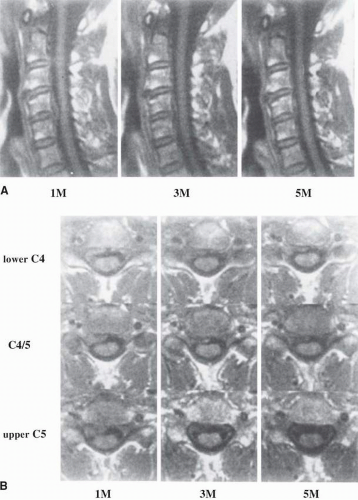
As there is a significant prevalence of such abnormalities in asymptomatic subjects, advanced cervical spine imaging in symptomatic individuals must be interpreted carefully to determine if a correlation exists between radiographic pathology and clinical findings (97, 98). Also of interest is the natural radiographic history of symptomatic disc pathology in the cervical spine. A study following “soft” disc herniations in 21 patients with radiculopathy found that the largest lesions decreased in size the most, with 16 of 21 lesions reducing in size by 35% to 100% at 15-month follow-up CT (99). In similar studies observing patients with serial MRI, 40% to 92% of patients demonstrated radiographic regression (100, 101). More lateral and extruded discs were observed to be more likely to resolve (Fig. 32-9).
Electrodiagnostics
Electromyography (EMG) studies can help to localize the level of a cervical radiculopathy and differentiate such a presentation from a brachial plexopathy, more distal entrapment, or peripheral neuropathic process. Such testing is not necessary in all patients presenting with cervical radicular syndromes. Electrodiagnostic studies might prove particularly useful in individuals with multiple levels of radiographic pathology whose physical examination findings are less conclusive in identifying the segmental pathology of clinical significance. In other cases, a patient might present with clinical findings suggestive of radiculopathy but without a clear radiologic correlate. In these cases, electrodiagnostic studies might similarly prove useful. The earliest abnormality that might be observed in the setting of motor root compromise is reduced voluntary recruitment observed during needle exam. Abnormal activity at rest in the form of positive sharp waves or fibrillation potentials, indicative of axonal loss, might not be observed until at least 18 to 21 days after the onset of a radicular syndrome (102).
EMG abnormalities have been shown to correlate well with myelographic abnormalities and operative pathology (103). Needle examination of seven limb muscles is likely adequate for the identification of segmental pathology (104). The segmental sensitivity and specificity of EMG abnormalities might only approach 67% and 50%, and EMG abnormalities can be appreciated at asymptomatic levels (105). The sensitivity and specificity of EMG abnormalities have not been studied against an agreed-upon gold standard. The false-negative rate of electrodiagnostic studies is likely higher than desired (106).
EMG abnormalities have been shown to correlate well with myelographic abnormalities and operative pathology (103). Needle examination of seven limb muscles is likely adequate for the identification of segmental pathology (104). The segmental sensitivity and specificity of EMG abnormalities might only approach 67% and 50%, and EMG abnormalities can be appreciated at asymptomatic levels (105). The sensitivity and specificity of EMG abnormalities have not been studied against an agreed-upon gold standard. The false-negative rate of electrodiagnostic studies is likely higher than desired (106).
A diagnosis of cervical radiculopathy is established in a patient when corroborative radiographic findings are observed in association with a suggestive pain distribution and/or a myotomal strength deficit, reflex abnormality, or sensory loss. If the cervical radiographs and clinical findings are less conclusive, electrodiagnostic studies might prove helpful in confirming a level of radicular pathology when a myotomal denervation pattern is observed. If radicular pain remains within the differential and these diagnostic criteria are not satisfied, a diagnostic selective nerve root block might be employed. Although the specificity and sensitivity of lumbar diagnostic injections have been described as ranging from 87% to 100% (107, 108), the utility of diagnostic selective cervical injections has not been as well-defined (109).
History
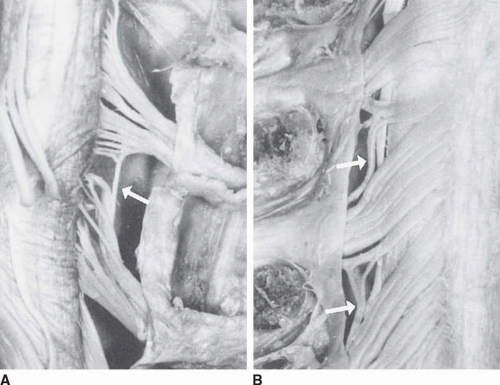
Patients with radiculopathy typically present with a chief report of pain, weakness, paresthesias, or a combination of sensorimotor deficits (65). The majority of patients describe cervical and upper-extremity pain that began without trauma or a particular inciting event (72, 73, 110). Most studies suggest that pain affects the upper limb more commonly than the neck, although most often both are involved (73, 79). Coughing, sneezing, or a Valsalva maneuver may lead to symptomatic worsening. The reports of pain might also incorporate the anterior chest wall, resulting in a syndrome of pseudoangina pectoris (67, 111, 112, 113). It is often difficult to determine the symptomatic level based on the patient’s pain description alone. The distribution of symptoms might not follow the classic dermatomal patterns as defined by Keegan and Garrett (114) or Foerster (115). The limitations of these mapping studies include the assignment of dermatomal distribution based predominantly on the loss of sensation or hyposensitivity arising from compressive injuries. Each of these classic studies describes considerable dermatomal overlap. Dermatomal pain patterns, arising from selective cervical spinal nerve stimulation, have recently been described and may be more representative of the pain and paresthesia patterns clinically observed in radiculopathy (116). Using fluoroscopically guided cervical nerve root stimulation, pain patterns were described after the stimulation of 134 roots in 87 subjects. Some of the highlights of this study included pain distribution distal to the elbow in only 14% of C5 stimulations, C6 affecting the ulnar hand in 67%, C7 uniquely resulting in anterior head symptoms and most commonly referring pain to the chest, and C8 affecting the thumb in 14%. No nerve root pain distribution was observed to be confined to the classically defined dermatomes in greater than 50% of stimulations (116) (Fig. 32-10). Some of the discrepancy between cervical dynatome and the more classic dermatomal maps might also be explained by intrathecal cervical dorsal root intersegmental anastomosis, which can be present in as many as 61% of individuals (117, 118, 119) (Fig. 32-11). Additionally, pain in radiculopathy might arise in part from a myotomal or myalgic pain component arising from ventral nerve root irritation (120).
Physical Examination
When examining the patient with suspected cervical radiculopathy, it is essential that the clinician remains mindful of extraspinal disorders that can mimic nerve root pathology. A brachial plexopathy or neuralgic amyotrophy with upper-trunk involvement can appear similar to a radiculopathy of C5 or C6 origin (121, 122, 123). More localized disorders affecting the upper limb can similarly result in regional pain, including subacromial
bursitis, lateral epicondylitis, and bicipital tendinitis (65). More distal neural entrapments affecting the median, radial, or ulnar nerve should also be considered. A median neuropathy at the wrist can result in pain extending as proximal as the cervical region (65). When cervical radiculopathy and myelopathy coexist, such as in amyotrophic lateral sclerosis and syringomyelia, the examiner should seek signs of possible combined cord and root compression (65, 124).
bursitis, lateral epicondylitis, and bicipital tendinitis (65). More distal neural entrapments affecting the median, radial, or ulnar nerve should also be considered. A median neuropathy at the wrist can result in pain extending as proximal as the cervical region (65). When cervical radiculopathy and myelopathy coexist, such as in amyotrophic lateral sclerosis and syringomyelia, the examiner should seek signs of possible combined cord and root compression (65, 124).
Muscle girth should be carefully inspected for any evidence of atrophy that might be consistent with a more chronic radiculopathy. Although C5 and C6 involvements can lead to wasting of the biceps and periscapular musculature, loss of triceps girth can be observed in the setting of a C7 radiculopathy. Atrophy of the hand intrinsics might be observed in patients with C8 or T1 pathology. Active cervical range of motion can be recorded and observed for symptom provocation in a particular plane. In particular, extension and ipsilateral side bending are more likely to result in a reproduction of radicular symptoms. Normal range of motion of the neck, using an inclinometer, has been estimated at 60 degrees of flexion, 75 degrees of extension, and 45 and 80 degrees of bilateral side-bending and rotation (125).
Manual muscle testing has been suggested to be a more sensitive means of isolating a level of segmental pathology than either reflex or sensory testing. Variations in the contributions of the spinal nerves to the cords and branches of the brachial plexus can lead to an altered pattern of muscular innervation in the upper limb and perhaps more perplexing motor testing findings (65, 74). Reflex testing of the upper extremity should include the bicep, tricep, brachioradialis, and Hoffman’s response. Lower-extremity reflex testing should also be included to rule out hyperreflexia or long tract signs. Gait should similarly be observed to rule out ataxia, which might be observed in the setting of cervical cord compromise.
Spurling’s maneuver incorporates rotation and side-bending of the head toward the affected side, and this can be combined with axial compression. The goal of this maneuver is to diminish the foraminal area and reproduce radicular symptoms (30). This neck compression test was initially defined by Spurling and Scoville in the 1940s at a time when cervical radicular syndromes were first recognized (126). This maneuver has been demonstrated to have a high specificity but a low sensitivity (110). Such maneuvers should be performed with caution in patients with a suspected radicular syndrome, as this could lead to further neural irritation. In those patients demonstrating a reproduction of radicular symptoms with active cervical extension or ipsilateral side-bending, this provocative test should be avoided (65). The shoulder abduction relief sign has the patient place the affected hand on the top of the head. This position of upper-extremity abduction reduces stress on the nerve root affected by a disc herniation and relieves pain (127, 128).
A complete exam should also include passive ranging of the shoulder and impingement maneuvers. Tinel’s testing at the supraclavicular fossa, elbow, and wrist and carpal tunnel stress maneuvers, such as Phalen’s testing and carpal compression, might reveal a proximal entrapment neuropathy or carpal tunnel syndrome. Observing for a pain response during palpation of the medial and lateral epicondyle can be helpful in ruling out a contributing focal tendonitis. Thoracic outlet maneuvers should also be considered to rule out a contributing lower-trunk plexopathy or dynamic vascular insufficiency, also referred to as “neurogenic” or “vascular” thoracic outlet syndromes. These regional exam techniques are included in the complete examination of a patient with suspected radiculopathy and might enlighten the examiner to a contributing musculoskeletal disorder or more distal neuropathic process.
Nonsurgical Treatment
Lees and Turner studied 57 patients with a diagnosis of cervical radiculopathy for a period of up to 19 years (35). Patients in this study pursued a variety of treatments, including rest, manipulation, therapeutic exercise, and soft-collar use. Of these 57 patients, 45% described one primary pain episode without a future symptomatic recurrence. Of the remaining patients, 30% described a continuation of mild symptoms, and 25% reported a persistence of significant symptoms or worsening. Of the 57 patients, 32 remained significantly improved, whereas 18 described their condition as static or symptomatic. In their series of patients with radiculopathy, none became myelopathic. Cervical collars appeared to correlate with initial symptomatic relief, but end results were similar among all patients studied.
A review of multiple retrospective and uncontrolled nonsurgical cervical radiculopathy studies revealed satisfactory outcomes in 80% to 90% of patients (73, 129, 130). Several randomized controlled studies in patients with lumbar syndromes have demonstrated an efficacy of nonsteroidal anti-inflammatory agents and muscle relaxants. Studies investigating the efficacy of oral corticosteroids have been inconclusive (131, 132). In a retrospective study of 26 consecutive patients treated nonsurgically, 20 achieved good to excellent results with an average of 2.3 years of follow-up. Oral analgesics, collars, traction, and physical therapy were employed in this treatment group. Twenty-two patients used oral steroids, and nine received epidural injections. Patients with extrusions were noted to fair better than those with contained protrusions (133). In a randomized prospective study, 81 patients were treated with anterior decompressive surgery, physical therapy, or collar use. The surgical group demonstrated significantly greater reductions in pain at 16 weeks, and both the surgical and physical therapy groups demonstrated improved functional abilities as compared with the collar group. No significant difference in outcome as measured by pain and functional profiles was observed between the groups at the final 12-month follow-up evaluation (134). In a prospective study of 155 patients with cervical radiculopathy, one third were treated surgically, whereas two thirds were prescribed medical treatment that included oral analgesics and steroids, traction, and epidural injection. At 1-year follow-up, significant improvements in pain and functional status were observed in both groups, with a greater degree of improvement in the surgical group. Twenty-six percent of the surgical group continued to experience severe pain (135).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree