Fig. 17.1
The stages of somitogenesis. Development occurs in a craniocaudal order. The more cranially placed somites (at the lower right of the figure) are further developed than those caudally placed (at the upper left of the figure). The stages in somitogenesis are given on the left of the figure; more detailed information is given on the right (This figure was published in Gray’s Anatomy, 40th Edition, Standring et. al., Development of the Back, 764, Copyright Elsevier (2008) [9]. Reproduction of this material is granted by Elsevier)
The sclerotome is the source of the vertebral column, ribs, tendons, and meninges, and the dermomyotome generates the vertebral and limb muscles, dermis, endothelial cells, and cartilage of the scapula blade [4, 5]. Each sclerotome has several portions: the central sclerotome (the main mass, close to the dermomyotome), the ventral sclerotome (close to the notochord), dorsal sclerotome, and lateral sclerotome (portions adjacent to the dermomyotome) [9] (Fig. 17.2).
A separate layer termed as the myotome is formed by the mesenchymal cells arising from the dorsomedial and ventrolateral borders of the dermomyotome [3] (Fig. 17.2). The cells produced by the myotome give rise to skeletal muscle dorsal to the vertebrae [9] (Table 17.1). At limb levels, cells from the ventrolateral edges of the dermomyotome loose their epithelial characteristics and migrate into the limb bud [9].
Differentiation of Muscle Cells
There are three types of muscle cells in the human body: smooth muscle, cardiac muscle, and striated muscle. Only the development of the striated muscle, which is one of the main interest areas of this book, will be discussed in this section.
Skeletal muscle of the trunk and limbs is derived from the myotomes of the somites [10]. As stated above, each somite has a myotome part. Each myotome divides into an epaxial (dorsal) division and a hypaxial (ventral) division [11]. These two divisions are innervated by the dorsal primary ramus and the ventral primary ramus, respectively, which are formed by the division on the spinal nerve that comes from the corresponding level of the developing spinal cord [11]. Even though muscles without an innervation will develop completely, a muscle that never had a nerve supply does not attain its full differentiation at the fiber level and disappears with time [2]. The myotomes and the muscles derived from them are listed in Table 17.1 [11]. The first myotomes begin to differentiate into skeletal muscles early in the fifth week, and nearly all the skeletal muscles are present in a fetus of 8 weeks [2].
Myotome | Structure |
|---|---|
Epaxial divisions of the myotomes | Extensor muscles of the neck, vertebral column, and lumbar region (deep muscles of the back that are attached to the vertebrae [7]) |
Sacral and coccygeal myotomes | Dorsal sacrococcygeal ligament |
Hypaxial divisions of the cervical myotomes | Scalene, prevertebral, geniohyoid, and infrahyoid muscles |
Thoracic myotomes | Lateral and ventral flexor muscles of the vertebral column |
Lumbar myotomes | Quadratus lumborum muscle |
Sacrococcygeal myotomes | Muscles of the pelvic diaphragm and probably the striated muscles of the anus and sex organs |
Ventral portions of the last six or seven thoracic myotomes | Rectus abdominis muscle [2] |
The structural units of skeletal muscle are called muscle cells or muscle fibers or myofibers [12]. The formation of new muscle fibers is termed as myogenesis. Prenatal myogenesis is divided into primary and secondary (fetal myogenesis), by which primary muscle fibers (during the embryonic stage) and secondary muscle fibers (during the fetal stage) develop respectively [13]. Myogenic precursors are derived from the cells that delaminate from the epaxial (adjacent to the neural tube) and hypaxial (adjacent to the lateral mesoderm) lips of the dermomyotome [7]. Myogenic precursor cells proliferate to increase their number. Under proper induction, myogenic precursors differentiate into myoblasts [13]. Myoblasts are mononucleated cells [2]. Under the influence of environmental signals, myoblasts align with each other, and their nuclei and cell bodies elongate in a direction parallel to the long axis of the embryo and proliferate by repeated mitotic divisions [2, 13]. Subsequently, they fuse with each other to form syncytia. Each syncytium turns into a tube (myotube) with continuous cytoplasm. The formation of myotubes begins at about the fifth week of embryonic development. The numerous nuclei within each syncytium are centrally located. Myofilaments of actin and myosin are laid down within the cytoplasm of each myotube and are oriented parallel to the long axis of the tube side by side. Thus, the myotube becomes a muscle fiber [2]. The myotubes become invested with external laminae during their development, and the external laminae segregate them from the surrounding connective tissue. The perimysium and epimysium layers of the fibrous sheath of the muscle are produced by fibroblasts; the endomysium is formed by the external lamina and reticular fibers [11]. The nuclei of the muscle fibers do not divide mitotically or amitotically. For the muscle fibers to grow in length, incorporation of new myoblasts into the syncytia is required [2]. The majority of muscle fibers are formed by secondary myogenesis. For this reason, the fetal stage is critical for skeletal muscle development [13]. Development of primary myotubes is autonomous, but the development of secondary myotubes, which form the majority of muscle cells of the adult, is dependent on innervation. Also, new muscle fibers cannot be generated in the absence of muscle contraction even if the nerves are present [14]. The persisting population of myoblasts found in close relationship to muscle fibers is the sources of subsequent generations of myotubes [2].
Development of Cartilage Tissue
The mesenchymal cells proliferate and condense in areas where the cartilage is going to form [15, 16]. These areas are termed chondrification centers [15]. Mesenchymal cells take round shape with round or oval nuclei and a low cytoplasm to nucleus ratio. The cells differentiate into chondroblasts, which secrete the extracellular matrix (type II collagen fibers, type IX collagen, and cartilage proteoglycan core protein) of the cartilage tissue [16]. There are three types of cartilages: hyaline cartilage (e.g., in synovial joints), fibrocartilage (e.g., in intervertebral discs), and elastic cartilage (e.g., in auricle of the ear) [15]. In areas of hyaline cartilage, continuous secretion of matrix separates the cells of condensed chondrification centers, thus taking the typical appearance of hyaline cartilage [16]. Hyaline cartilage is the most widely distributed type of cartilage and an example is the articular cartilage [15]. In areas of fibrocartilage formation, many of the cells differentiate into fibroblasts and secrete collagen. Chondroblastic activity appears only in isolated groups or rows of cells which become surrounded by dense bundles of collagen fibers secreted by the fibroblasts [16]. Fibrocartilage is found in intervertebral discs [15]. In areas of elastic cartilage formation, elastic fibers are secreted by the chondroblasts [16]. Elastic cartilage exists in auricles of the external ears [15].
The condensed mesenchyme surrounding the developing cartilage differentiates into a bilayered perichondrium [16]. The articular cartilage is not surrounded by perichondrium [2]. The cells of the outer layer of the perichondrium differentiate into fibroblasts and secrete a dense collagenous matrix. The cells of the inner layer differentiate into chondroblasts or prechondroblasts, which are in a resting state [16].
Development of Bone Tissue
Two types of bone formation (ossification) occur in the embryo. In intramembranous ossification, the mesenchyme condenses and becomes highly vascular [15]. Some cells differentiate into osteoprogenitor cells [16]. Osteoprogenitor cells proliferate around the branches of the capillary network and differentiate into osteoblasts (bone-forming cells) [15, 16]. Osteoblasts begin to deposit osteoid (unmineralized matrix), in which calcium hydroxy apatite is later deposited. As the osteoblasts deposit the matrix around their cell membrane, they are trapped and become osteocytes. During this process and afterwards, osteocytes remain connected to each other via their fine processes [16]. The space occupied by the osteocytes is called lacunae and the tubular spaces occupied by their processes are called canaliculi [15, 16]. As the bone spicules grow in size by the addition of newly synthesized matrix, they form a network of trabeculae [16] (Fig. 17.3).
In endochondral bone formation, first, a model of hyaline cartilage is formed in the area where the bone is to form [15]. The deep layers of the perichondrium around this model contain osteoprogenitor cells. These cells differentiate into osteoblasts [16]. Osteoblasts form a layer of bone tissue that surrounds the central shaft of the cartilage model (i.e., the diaphysis of the future bone) [15]. This layer of the bone is termed as the bony collar (periosteal bone) and is the first sign of ossification. From this point on, the role of the perichondrium has changed and it is now the periosteum [17]. The bony collar is thought to act as a diffusion barrier which impedes nutrient movement from the capillary network residing outside the cartilage model and thus triggers the events in endochondral ossification by virtue of nutrient deprivation [18, 19]. Concurrently with the formation of the bony collar, the chondrocytes within the center of the cartilage model begin to enlarge, their cytoplasms become vacuolated, and they accumulate glycogen [16, 17]. The matrix between these hypertrophic cells is compressed and resorbed and thus, perforated septa of thin irregular cartilage plates are formed. The cells begin to synthesize alkaline phosphatase, and the surrounding cartilage undergoes calcification [17]. The cells degenerate and leave behind enlarged lacunae. Blind-ended capillary sprouts (osteogenic or periosteal buds) originating from the periosteum invade the lacunae [16]. Osteogenic buds are accompanied by osteoprogenitor cells, and osteoclasts [16]. The blood vessels branch and project toward the borders of the primary ossification center [20]. Osteoprogenitor cells differentiate into osteoblasts, which attach themselves to the delicate residual walls of calcified cartilage and begin to deposit osteoid. A continuous lining of the bone forms over the cartilage residue [16]. A network of trabeculae forms by the addition of new osteoid over the previously formed spicules with cartilage cores (mixed spicules) [17]. At the same time, remodeling and removal of cartilage residues takes place by the action of osteoclasts [16, 17, 20] (Fig. 17.4). This area of the long bone, which is the first place of bone formation, is termed as primary ossification center [16, 17, 20]. Primary ossification center enlarges to the both ends of the bone until it reaches the future area of epiphyseal cartilage. Epiphyseal cartilages remain cartilaginous and persist until longitudinal growth ends in early adulthood [17]. The bony collar also extends to the both ends of the shaft of the long bone [16]. It is soon remodeled into compact bone. Secondary ossification centers appear in epiphyses of long bones shortly after birth [17]. In order for the embryo to grow in length, the cartilaginous model does not entirely turn into a bone. As the ossification front reaches near the ends of the cartilage model, the chondrocytes near the ossification front proliferate prior to undergoing hypertrophy and push out the cartilaginous ends of the bone [21]. These areas between the epiphyses and diaphyses of long bones are called epiphyseal growth plates [20]. Epiphyseal growth plate is formed of five zones: zone of reserve cartilage, zone of proliferation, zone of hypertrophy, zone of calcified cartilage, and zone of resorption. As ossification occurs by the events that occur in the last three zones, cartilage cells in the reserve cartilage that are nearest to the zone of proliferation proliferate and maintain the thickness of the proliferation zone [17].
The development of skeletal elements starts along a common pathway which diverges into osteogenic or chondrogenic programs depending on the nature of their local microenvironment [22] (Table 17.2). The long bones other than the clavicle are of endochondral origin [2].
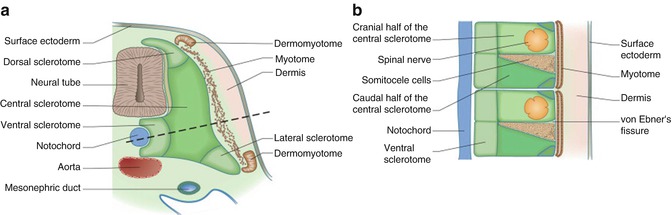
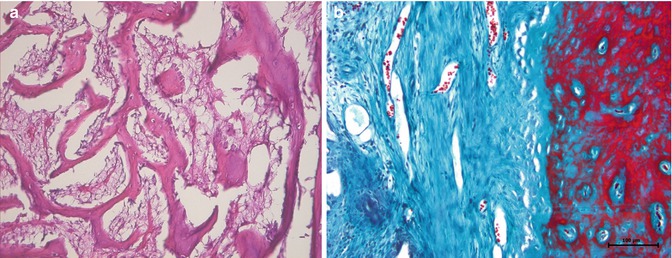

Fig. 17.2
Later development of the sclerotome. Transverse (a) and longitudinal (b) section through dotted line indicated in (a) showing the sclerotomal subdivisions (This figure was published in Gray’s Anatomy, 40th Edition, Standring S. et. al., Development of the Back, 765, Copyright Elsevier (2008) [9]. Reproduction of this material is granted by Elsevier)

Fig. 17.3
Intramembranous ossification. (a) Acidophilic bone trabeculae lined by osteoblastic cells, hematoxylin and eosin 200×. (b) Mature calcified bone is in red. Collagenous connective tissue that surrounds the osteoid is in blue, Masson’s trichrome 200×
Development of the Joints
The joints begin to develop during the sixth week. Areas of condensed mesenchymal cells are observed between the neighboring developing bones [15]. These areas are termed interzonal mesenchyme and are the sites of future joints. In areas where fibrous joints will form, the interzone is converted into collagen [23] (Fig. 17.5). In synchondroses, the interzonal mesenchyme differentiates into hyaline cartilage, and in symphyses, it differentiates into fibrocartilage. In developing synovial joints, an intermediate zone appears in the interzonal mesenchyme and splits it into two dense layers [23]. The interzonal mesenchyme becomes cartilaginous like the developing bones, but cavitation takes place, and thus the joint cavity is formed [2, 23]. The mesenchyme around the cavity forms the joint capsule. The developing joints resemble adult joints by the end of the eighth week [15].
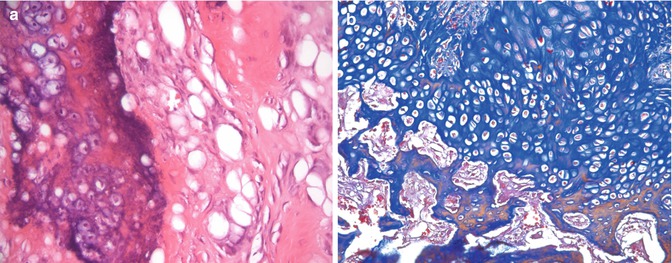





Fig. 17.4
Endochondral ossification. (a) Calcified cartilage is basophilically stained at the left side; new bone spicule is in pink (acidophilic) at the right side of the micrograph, hematoxylin and eosin 200×. (b) The cartilage is in blue at the upper part, and the new remodeling bone trabeculae with removal of cartilage residues are reddish at the bottom, Mallory trichrome 200×
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








