CHAPTER 94 Complications of Spinal Surgery
Pathoanatomy of Neural Injury
The spinal cord lies protected within the spinal canal. The nerve roots exit segmentally through their corresponding neural foramina. Within the spinal canal, the pedicle is a consistent anatomic landmark that can be used to locate the exiting nerve roots and is useful especially in the presence of anomalous anatomy. In the upper cervical spine the hypoglossal nerves (cranial nerve XII) lie 2 to 3 mm lateral to the midpoint of the lateral masses of C1. They are at risk for injury from overpenetration of C1-C2 transarticular screws, which can result in tongue deviation toward the side of injury and dysarthria.1,2 The C2 nerve root exits dorsal to the C1-C2 facet joint and is at risk for injury during placement of C1 lateral mass screws, which can result in pain or paresthesias in the C2 dermatome (i.e., occipital sensation).3 In the subaxial cervical spine the nerve roots exit anterolaterally through the neural foramen and lie within a groove immediately anterior to the superior articular processes, placing them at risk for injury from improperly placed cervical lateral mass and pedicle screws. In anterior approaches to the cervical spine, dysphagia or dysphonia can result from injury to the pharyngeal plexus during approaches to C2-C5, the hypoglossal during approaches above the level of C3, the superior laryngeal nerve during exposure of the C3-4 level, and recurrent laryngeal nerves during approaches to C5-T1.4 The right recurrent laryngeal nerve passes around the subclavian vessels and then ascends in the neck, coursing medially at a variable level (typically C6 to C7) along with the inferior thyroid artery to its destination in the larynx. The left recurrent laryngeal nerve, in contrast, passes around the ligamentum arteriosum at the aortic arch and then predictably ascends within the tracheoesophageal groove, where it remains relatively protected before its destination in the larynx. This distinction is most relevant in surgical approaches to the lower cervical spine, where the variability in the course of the right recurrent laryngeal nerve is greatest, making it at increased risk for injury during dissection. The cervical sympathetic chains lie ventral to the longus colli and course most medially at C5-6, where they are at greatest risk for injury.5 Injury can result in Horner syndrome of ptosis, meiosis, anhidrosis, and enophthalmos. In the thoracic spine the ventral nerve roots continue as the intercostal nerves and lie with the costal groove on the undersurface of the rib. They are at risk for injury during surgical dissection in this area. In the lumbar spine the nerve roots lie along the inferomedial edge of the pedicle and are at risk of injury from medial and inferior pedicle breaches during pedicle screw placement. The lumbar sympathetic chain lies anterior to the psoas muscle and can be injured during retroperitoneal approaches to the lumbar spine manifesting as patient complaints of contralateral foot coolness. The superior hypogastric sympathetic plexus is at risk for injury during approaches to the L5-S1 disc space.1,2 Injury can result in retrograde ejaculation and infertility from lack of proper sphincter control, which misdirects the ejaculate into the bladder instead of through the urethra. This is less of a concern at upper lumbar levels because the fibers of the plexus lie on the anterior aortic wall and only become immediately prevertebral at the level of the L5-S1 disc space. The lateral femoral cutaneous nerve, which provides sensation to the anterolateral thigh, may be injured during harvesting of bone graft.6 Although the nerve typically passes anterior to the anterior superior iliac spine (ASIS), variants may cross posterior to the ASIS, placing the nerve at risk for injury with dissection within 3 cm posterior of the ASIS. Injury to the nerve can result in painful neuromas and meralgia paresthetica. The superior cluneal nerves, cutaneous branches of L1-L3 involved in sensation to the buttock, may be injured during harvesting of bone graft from the posterior iliac crest with dissection more than 6 cm lateral to the posterior superior iliac spine.7
Intraoperative Complications and Their Prevention
Preoperative Patient Evaluation
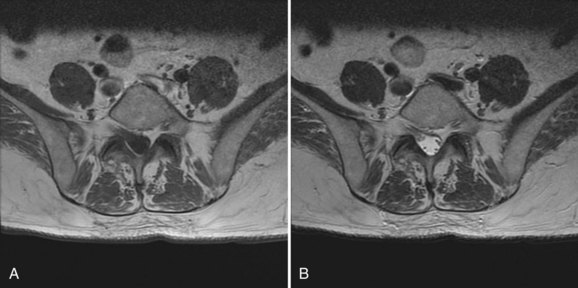
Preoperative evaluation of the patient begins with an accurate diagnosis obtained after a thorough history and physical and review of imaging studies. Preexisting neurologic deficits should be identified, and a baseline neurologic examination obtained and accurately documented. These should be communicated with other health care providers involved in the care of the patient. The preoperative imaging studies should be thoroughly reviewed for anatomic anomalies that may increase the chance for neurologic injury. Dysraphic states can increase the risk of durotomy and complicate exposure. Nerve root anomalies increase the risk of injury if not identified preoperatively (Fig. 94–1). Areas of significant neurologic compression increase the risk of injury to neural structures from surgical decompressive maneuvers because there is less room for error. In these situations, significant stenosis results in loss of the normal subarachnoid space. Normally, the subarachnoid space provides a protective buffer between the dura mater and neural structures. Loss of the normal subarachnoid space increases the chance of contusing neural tissue with surgical maneuvers performed immediately adjacent to the thecal sac (e.g. use of a Kerrison rongeur, sublaminar wiring, epidural bipolar electrocautery during anterior cervical discectomy). Abnormal osseous anatomy (e.g., abnormal pedicle morphology, compressive osteophytes, ossified ligamentum flavum) can complicate decompression, instrumentation, or both and should be identified before surgery because these affect intraoperative tactics. Patients should be instructed to preoperatively discontinue anticoagulant medications to minimize intraoperative and postoperative blood loss and the potential for epidural hematoma formation.
In addition to the specific neurologic risks of a surgical approach, patient factors (e.g., gender, history of prior surgery, preexisting neurologic deficits) and their tolerance for specific injuries may play a role in determining the risk-to-benefit ratio of a given approach; thus it is important to have a preoperative discussion with patients regarding the risks for neurologic injury and determine how willing they are to accept these risks. For example, retrograde ejaculation and male infertility can result from injury to the superior hypogastric plexus during an anterior lumbar approach with transabdominal approaches at especially increased risk in comparison with retroperitoneal approaches.8 Although blunt dissection anterior to the L5 and S1 vertebrae can decrease the chances for injury, it cannot eliminate this risk. Although preoperative cryopreservation (i.e., sperm banking) is an option to retain the possibility of procreation in the event of postoperative infertility, a young male interested in having children may not want to bear this risk. History of prior surgery can also significantly alter the risk-to-benefit ratio of using a given approach because operating through a prior surgical incision (especially during anterior approaches to the spine) considerably increases the risk for complication. This increased risk may be acceptable, however, if the relevant structures have already been compromised. For example, in revising an anterior cervical fusion in a patient with acquired left recurrent laryngeal nerve palsy from a previous left-sided approach, repeat use of a left-sided approach may be preferable because this minimizes the risk of bilateral recurrent laryngeal nerve injury and consequent exacerbation of postoperative dysphagia and/or dysphonia.
Positioning
The surgical and anesthetic teams should work together to ensure proper patient positioning to minimize the chances of neurologic injury. Special attention should be paid to positioning of the neck and limbs with proper padding of superficial nerves. Abnormal cervical postures should be avoided. Patients should be positioned with their heads in a neutral or slightly flexed position without excessive rotation because neurologic injury can occur from cervical extension in myelopathic patients, which narrows the internal diameter of the spinal canal and from excessive neck rotation to one side, which narrows the ipsilateral cervical neuroforamina. Case reports exist of excessive neck rotation resulting in carotid artery occlusion and subsequent stroke.9 In cervical spine surgery, the shoulders are often depressed and taped in position to improve radiographic visualization. If excessive, this can result in a traction injury to the brachial plexus. Placing a patient into the prone position requires a coordinated effort directed by the individual controlling the head. The persons receiving the patient have the important task of controlling the rate of descent of the patient onto the operating room table. In patients with an unstable spine, gentle inline traction can help maintain alignment. The limbs should be positioned to minimize the potential for compression or traction injury to the peripheral nerves and the brachial plexus. Extra care should be taken in patients with stiff shoulders, morbid obesity, and other circumstances that mitigate against correct positioning of the limbs.
Proper positioning also plays an important role in the prevention of ocular complications. Postoperative visual deficits have a reported incidence as high as 0.1% in spinal surgery in the prone position but have also been described in supine patients.10 The most common cause (>80% of cases) is ischemic optic neuropathy associated with surgical blood losses greater than 4 L with a hypotensive event or relative hypotension over an extended period, either from blood loss or deliberate hypotension to minimize blood losses.11 Visual deficits can also result from central retinal artery or vein occlusion from direct pressure to the globe, resulting in increased intraocular pressure and decreased retinal blood flow through the central retinal artery. Transient cortical blindness can result from an isolated stroke that affects the visual cortex typically from an embolic phenomenon or hypoperfusion. In contrast to ischemic optic neuropathy and central retinal vessel occlusion, which are typically irreversible, most cases of cortical blindness resolve. Although the risks of these complications cannot be eliminated, they can be minimized through prevention of direct pressure to the globe (e.g., Mayfield three-point head holder in posterior cervical spine surgeries) and appropriate patient-specific management of hemodynamic parameters with measures aimed at reducing central venous pressure (e.g., reverse Trendelenburg positioning, minimizing abdominal compression).12 These considerations are especially important in high-risk patients (i.e., diabetes, hypertension, glaucoma, large estimated blood losses, systemic hypotension, and long operative times).
Surgical Technique
There is no substitute for good surgical technique and knowledge of surgical anatomy in the prevention of neurologic injury. Proper handling of each surgical instrument is paramount because, if mishandled, any instrument has the potential to cause injury. Instruments should not be passed over an open spinal canal, and all instruments introduced into the wound should be checked to make sure they are not going to come apart and risk blunt or penetrating injury to the thecal sac (e.g., screwdriver shafts should be checked to ensure they are seated within their handles). When possible, instruments should be held with two hands when working around vital structures, with the hand closer to the patient resting against the patient for stability and to prevent “plunging.” In addition, instruments should be directed away from important structures when possible to avoid injury due to inadvertent “past-pointing.” Instruments should be properly sized for the task at hand. Instruments with cutting surfaces should be kept sharp because this allows more control as less force is necessary. A thorough understanding of the local anatomy and familiarity with the instrument allows proper orientation of the instrument in relation to structures at risk. For example, directing the path of a Kerrison rongeur in line with the nerve root can help decrease the chance of inadvertently biting the nerve root during a foraminotomy. When possible, protecting the path of cutting instruments with a cotton pledget or with gentle retraction can be helpful to avoid injury; however, excessive compression or retraction of the neural elements should be avoided. Osteotomes may be useful in cases of severe stenosis because the overlying compressive structures (typically an overgrown facet joint) can be removed without having to introduce an instrument into an already tight spinal canal. Running motorized burrs at high speeds decreases the chances of skipping on sclerotic bone. Drills should ideally be started perpendicular to the bone to prevent skipping; alternatively, a starting indentation may be created with an awl or burr. Past-pointing with the drill should be avoided through the use of tactile and auditory cues to identify when cortical penetration is imminent. It is common to lose focus toward the end of a long spine procedure, especially during the more routine portions of the procedure after the challenging sections have been completed. This is the time when it is most important to remain vigilant because lapses in concentration can risk iatrogenic injury. An inadvertent slip of an instrument into an open spinal canal during final torquing of set screws, for example, is all it takes to ruin an otherwise technically well-executed procedure. Proper placement of retractors is essential to avoid injury. The assistant holding the retractor ideally should have adequate visualization and be free from other duties in order to focus solely on safe retraction. In general, the thecal sac should not be retracted at cord levels. In the lumbar spine at the level of the cauda equina, the thecal sac may be retracted for improved visualization, though excessive retraction risks neurologic injury, especially before decompression in the setting of stenosis. Excessive or poorly controlled intraoperative bleeding risks postoperative epidural hematoma and thecal sac compression. Excessive bleeding should be controlled before closure and drains used when appropriate. Hemostatic agents (e.g., gel foam) and bone wax can create neurologic compression if improperly or inadvertently placed.13
Neuromonitoring
Historically, many intraoperative neurologic complications were not identified until the patient awoke from surgery with a neurologic deficit. Now, with the advent of useful neuromonitoring techniques, perturbations in neural transmission can be identified early, allowing for timely intraoperative correction and mitigation of potential postoperative neurologic deficits. Neuromonitoring is most useful when there is considerable risk of neurologic injury from either patient positioning, planned operative maneuvers, or when the surgical plan calls for significant forces to be applied to the spinal column (e.g., deformity correction).15–17
tcMEPs
TcMEPs allow for a functional evaluation of the motor pathway including the anterior or ventral corticospinal tracts of the spinal cord, plexus, and peripheral nerve. Intermittently applied transcranial electrical stimuli result in evoked potentials that can be recorded either directly from the spinal cord or indirectly from muscle. Direct recording from the spinal cord via electrodes is not commonly used but can be useful for intradural procedures. More commonly, recordings are obtained indirectly from the muscle as compound muscle action potentials (CMAPs) via implanted electrodes. Transcranial MEPs should be distinguished from neurogenic MEPs, an older, less reliable technique that, despite its name, reliably monitors only sensory pathways.18 Transcranial MEPs are a useful complement to SSEPs, allowing for monitoring of both the ventral and dorsal spinal cord tracts. They are sensitive to changes in perfusion, which allows them to be used to titrate the degree of hypotension to spinal cord perfusion. Transcranial MEPs are more sensitive and respond more rapidly than SSEPs to changes in neural transmission, potentially increasing the window of opportunity for intervention in the face of neural injury.17,19
EMG
Electromyography (EMG) is used to evaluate nerve root integrity. There are two types in routine use, mechanically elicited EMG (meEMG) and electrically elicited EMG (eeEMG). MeEMG (also known as spontaneous or free-running) provides continuous monitoring of spinal nerve roots for injury. When a monitored spinal nerve root sustains microtrauma, the subsequent depolarization results in motor unit potentials in the corresponding innervated muscles that can be recorded via surface electrodes. Consequently, meEMG can be useful during placement of implants (e.g., pedicle screws) or when working in close proximity to a nerve root (e.g., posterior cervical foraminotomy), where continuous monitoring for nerve root microtrauma can alert the physician to injury. eeEMG (also known as stimulus-evoked or triggered) is used for intraoperatively evaluating implant placement (typically pedicle screws). eeEMG, in contrast to meEMG, is a static, stimulus-dependent technique and is not able to provide information over the course of an entire surgical procedure. Intermittent stimulation of an implant is performed while the corresponding “at-risk” nerve roots are monitored for signal transmission. Because cortical bone has a high resistance to electrical current flow in contrast to soft tissues, in the absence of a pedicular breach, stimulation of a pedicle screw should not result in signal transmission until the current exceeds an incompletely defined threshold (at our institution we use 10mA).20 When a breach is present, less current (≤5mA) is necessary for signal transmission because the path of least resistance (i.e., the soft tissues accessible via the breach) is taken. Readings between 5 and 10 mA are a gray zone; other factors need to be considered including, but not limited to, the confidence in the accuracy of screw placement, whether the screw placement is harmoniously aligned with the adjacent screws, the importance of the screw to the overall stability of the construct, and the degree of osteopenia. When eeEMG suggests a breach, unless irrefutable evidence is available (e.g., presence of a laminotomy with the ability to palpate and inspect the pedicle within the canal directly for evidence of breach), we would tend to remove the screw, check for a breach, and reposition the screw or opt to leave the screw out. When chronic compression of motor nerve roots exists, these previously mentioned thresholds can be elevated, resulting in false-negative results. In fact, some authors recommend a heightened level of concern when eeEMG testing returns a value less than 60% in comparison with the other screws regardless of absolute value.21 False positives are not infrequent and are an issue because of the need to remove the screw to check for a breach, which can lead to decreased screw purchase on reinsertion.
The amplitude, latency, and waveform morphology criteria for actual or impending neurologic injury with these aforementioned techniques are not clearly defined in the literature. Amplitude change appears to be the most relevant because significant injury is unlikely to occur without it. The most common criterion defines a significant change as greater than or equal to a 50% reduction in amplitude.22 Clinically relevant changes are typically abrupt and associated with a corresponding increase in latency. In contrast, isolated latency changes in the absence of amplitude alterations are often one of a variety of benign evoked potential alterations that can be misinterpreted as actual or impending neurologic injury.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree